Pulmonary tuberculosis in Patna, India: Durations, delays, and health care seeking behaviour among patients identified through household surveys
- DOI
- 10.1016/j.jegh.2017.08.001How to use a DOI?
- Keywords
- Delays; Pathways to care; Patna; Private providers; Pulmonary tuberculosis
- Abstract
Background: Delays in accessing effective health care plays a pivotal role in increasing Tuberculosis (TB) transmission within the community. Patna, North India, with high levels of poverty and weak public health system, faces huge challenges for achieving effective TB control. The study aims to determine delays that occur from onset of TB symptoms until initiation of pulmonary TB (PTB) treatment among patients in Patna.
Methods: Of the 109 self-reporting TB patients identified through an active household survey, 64 PTB patients were interviewed. First care seeking, TB diagnostic and treatment initiation durations were calculated and delays defined for new and retreatment patients and minors and adults. Outliers exhibiting extreme delays were additionally identified.
Results: A cross sMean total pathway duration for TB care was 40 days, with diagnostic duration contributing to 58% of the duration. No significant differences were noted between new and retreatment patients. Minors, comprising of 30% of total PTB patients accessed care faster than adults, but showed significantly higher diagnostic duration (38 days vs. 17 days). Preference for private sector, chemists and allopaths was seen throughout the pathway.
Discussion: Patna requires a more effective harnessing of the private sector augmented with reliable diagnostic investigations and a focus on quality.
- Copyright
- © 2017 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
India contributes almost 30% of the global Tuberculosis (TB) burden [1] with prevalence rate of 195 per 100,000 population nationally [2]. Despite efforts to achieve 70% case detection, the Revised National TB Control Programme (RNTCP) is unable to control the epidemic. Approximately 2.8 million TB patients, of the estimated annual global incident TB patients of 10.4 million in 2015 are reportedly from India [1]. Hence for effective control of TB, early care-seeking, accurate diagnosis and prompt initiation of treatment are paramount [3,4].
Recognizing the need to shorten patient pathways to TB care, a Private Provider Interface Agency (PPIA) strategy was planned to be introduced [5] before which, baseline evaluation studies were undertaken in 2014 in Mumbai and Patna. The baseline study conducted in the urban slums of Mumbai showed that patients experienced delays beyond two months from the onset of symptoms to first care seeking, diagnosis and treatment initiation [6]. It further demonstrated that Mumbai, with a well established public health sector and more than 60% population living in slums [7], saw TB patients switching between the private and public sectors in all three stages of care [6]. In this paper we present the findings from the baseline study conducted in Patna.
Patna with a population of 2.04 million [8], presents a different landscape from Mumbai. Burdened with an estimated notification rate of 77 per 100,000 population [9], the city is perceived to have a weak public health system with the overt presence of an unregulated private sector for TB care (World Health Partners, personal communication). Thus Patna presented an opportunity to understand the influence of the private sector in shaping the access to care pathways of TB patients.
Previous studies in India on patient pathways [10–12] have bypassed locations such as Patna. Additionally, most previous studies [13–15] have been facility-based in contrast to the present study, which was undertaken through active community participation and captured information on pathways of self-reported pulmonary TB patients.
2. Method
2.1. Study design and sampling
The Municipal wards in Patna city with the highest reported proportion of TB patients were identified through digital mapping. A community-based active survey was carried out in households (HH) of the three municipal corporations between May and August 2014 to identify self-reporting pulmonary TB patients. Subsequently in-depth interviews were conducted between May and September 2014. Three attempts were made to contact all those identified through the HH survey either via telephone and/or house visit to secure an interview. Patients of all ages and genders, diagnosed with pulmonary TB, or receiving anti-TB treatment (ATT) in the six month period prior to the interview, were included for in-depth interviews. Extra-pulmonary TB patients, patients diagnosed or completed ATT more than six months prior to the interview, patients not consenting for interview, and those too ill to speak, hospitalized or without a guardian, were excluded from the study. All interviews conducted by trained, local field researchers with social sciences background were supervised by qualified and experienced Public Health researchers. These were conducted within a week of their identification by the HH survey. The key domains of the interview schedule included patient’s general information, socio-demographic profile, health seeking behaviour and the pathway to TB diagnosis and care, with detailed enquiry about every provider accessed.
Among 109 TB patients identified through the initial HH survey, 64 were included in the final sample for in-depth interviews. Reasons for not including the remaining 45 patients were: a) extra-pulmonary TB patients – 13, b) TB history beyond the stipulated recall period of 6 months – 14, c) unavailability – 9, d) refusals for interview – 9.
2.2. Data collection and quality check
The in-depth interview schedule, information sheet and informed consent forms were developed in English, translated into Hindi (local language) and then back translated to check for consistency. All interviews were conducted in Hindi or the preferred local dialect, by two researchers, viz. the main interviewer and note taker. Interviewed patients were compensated monetarily (USD 4.5) for their participation at the end of the interview. On an average, these interviews lasted for sixty to ninety minutes. Interviews were audio recorded after consent from the respondent. Confidentiality on names and addresses was maintained throughout reporting.
In interest of a stringent quality check, interviews were reviewed using audio files, supporting documents and quantitative sheets at three levels:
- 1)
Peer review of interviews by a second team of researchers;
- 2)
Review of 25% of interviews by two senior researchers;
- 3)
Review of 10% of interviews by a study consultant.
2.3. Data management and analysis
Quantitative data were filled on code sheets by field researchers at the end of the interviews. These were cross checked for errors and the codes entered in CSPro v5. The data were then exported into SPSS v19 (SPSS, Chicago Inc, IL, USA) and analyzed.
Patients were analyzed based on a) age (minors vs. adults) wherein minors were defined as patients below 18 years b) past TB treatment (new vs. retreatment) to assess their modulation of pathways.
Since outliers constituted more than 10% of the patients for each segment of the pathway, differences were compared using t-test as opposed to a non parametric test.
2.4. Operational definitions
Total patient pathway duration was defined as the time interval between onset of symptoms suggestive of TB and the initiation of ATT. Duration beyond 35 days was considered as “total patient pathway delay”.
First care seeking duration was defined as the time interval between the onset of symptoms suggestive of TB and the first contact with any health care provider for the same. Duration beyond 15 days was considered as “first care seeking delay” as per STCI guidelines [16].
Diagnostic duration was defined as the time interval between first care seeking and receiving PTB diagnosis for the first time. Duration beyond 15 days was considered as “diagnostic delay” as per RNTCP guidelines [17].
Treatment initiation duration was defined as the time interval between the diagnosis of PTB and the initiation of TB treatment. Duration beyond 7 days was considered as “treatment initiation delay” as per RNTCP guidelines [17].
Outliers were patients with extreme delays computed on the median value of the delay segment of the pathway duration (in days). Thus outliers for first care seeking delay were those who had delays of 26 days or more, those for diagnostic delay had delays of 40 days or more and those for treatment initiation delay had delays of 15 days or more.
2.5. Ethical consideration
The study was approved by the Institutional Ethics Committee of the Foundation for Medical Research, Mumbai, India (FMR/IEC/TB/01/2013).
3. Results
3.1. Profile of patients
As depicted in Table 1, among the 64 PTB patients interviewed, 30% (n = 19) were minors. Proportion of gender among minors (males – 42% and females – 58%) and adults (males – 52% and females – 48%) were not significantly different. Fifty-six percent patients (n = 36) were not contributing to the family income at interview and 34% patients (n = 22) were illiterate. Twenty-three percent patients (n = 15) admitted to a past history of TB, while 33% patients (n = 21) reported having some form of addiction. Nine TB patients (14%) reported co-morbidities: diabetes, hypertension and fibroids.
| Age (years) | n | % |
| ≤17 | 19 | 30 |
| >18 | 45 | 70 |
| Gender n (%) | ||
| Male | 33 | 52 |
| Female | 31 | 48 |
| Occupation n (%) | ||
| Unemployed | 6 | 9 |
| Salaried | 9 | 14 |
| Self employed | 10 | 16 |
| Daily wage/casual | 6 | 9 |
| Housewife | 17 | 27 |
| Students | 13 | 20 |
| Not available | 3 | 5 |
| Education | ||
| Illiterate | 22 | 34 |
| Literate but no formal education | 7 | 11 |
| Primary (< 4th standard) | 7 | 11 |
| Secondary (< 9th standard) | 15 | 23 |
| Senior secondary (SSC/HSC) | 8 | 13 |
| Graduate and above | 3 | 5 |
| Not applicable | 2 | 3 |
| Classification | ||
| New (no history of TB) | 49 | 77 |
| Retreatment (past history of TB) | 15 | 23 |
| Use of addictive substances | ||
| Yes | 21 | 33 |
| No | 43 | 68 |
| Type of addictive substances consumed* | ||
| Cigarette/beedi | 4 | |
| Khaini/tobacco | 6 | |
| Alcohol | 16 | |
| Co-morbidity | ||
| Yes | 9 | 14 |
| No | 54 | 84 |
| Not available | 1 | 2 |
| Type of co-morbidity# | ||
| Diabetes | 5 | |
| Hypertension | 3 | |
| Others | 1 | |
some patients had more than one addiction.
some patients had more than one co-morbidity.
Patient characteristics (N = 64).
3.2. Initial symptoms as reported by patients
Cough was the most frequently reported initial symptom, followed by other cardinal symptoms such as fever and hemoptysis as seen in Fig. 1. Other symptoms included non-TB related symptoms such as leg and abdominal pain, diarrhea etc.
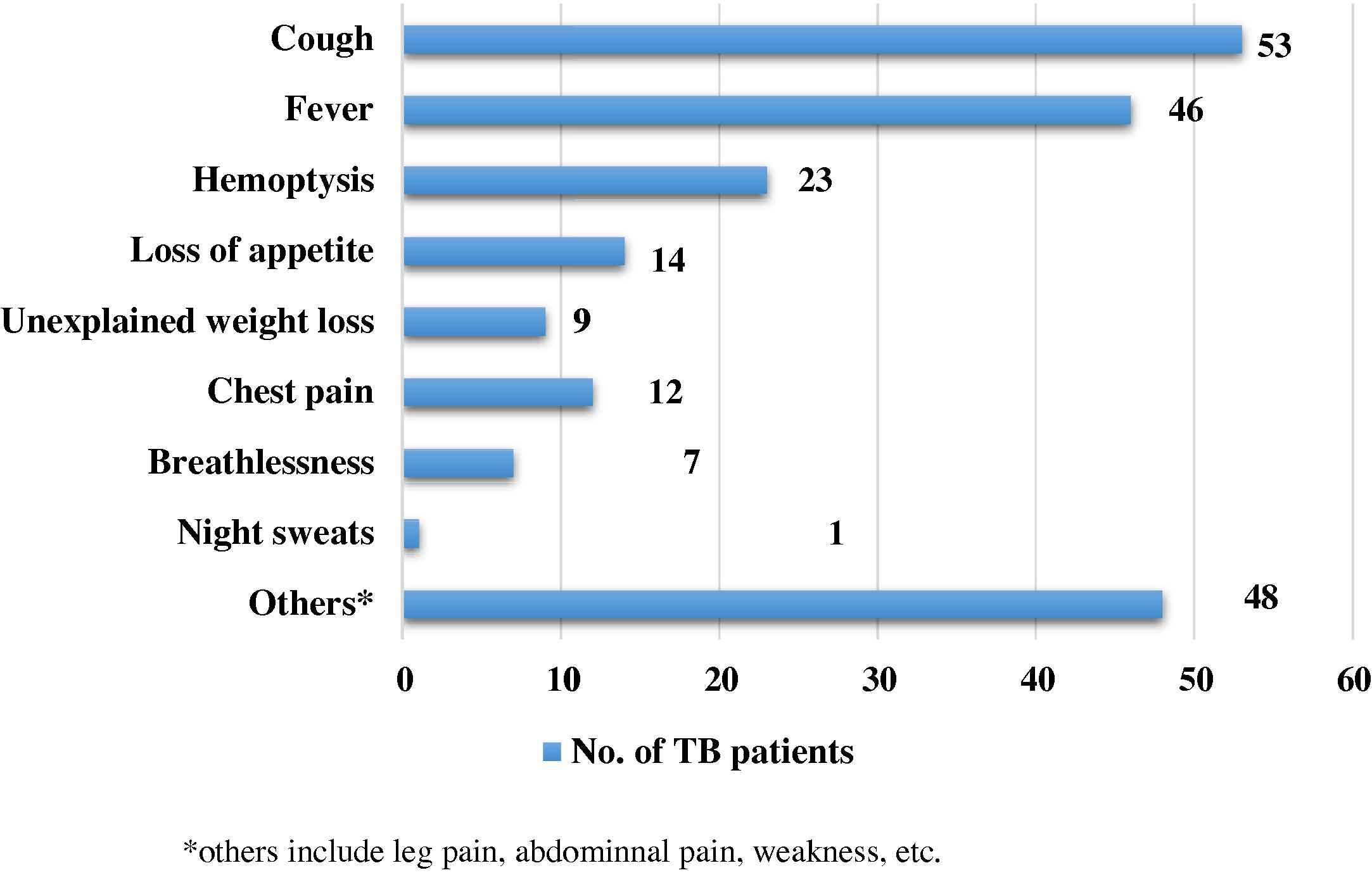
Symptoms reported by patients at the onset of the disease. The figure depicts the initial distressing symptoms experienced by TB patients.
3.3. First point of care
The first point of care after the onset of symptoms was predominantly the private sector. Ninety-four percent (n = 60/64) patients first sought care in the private sector. Among these 60 patients, 55% (n = 33) first sought care from allopaths, followed by chemists (n = 16; 27%) while 5% (n = 3) approached non-allopaths and one patient approached a compounder. Seven patients (12%) were unaware of the qualifications of their providers. Government hospitals were the first point of care for all four patients who approached the public sector.
3.4. Total patient pathway: durations and delays
The mean total patient pathway duration was 40 days (range: 1–164). This was not significantly different for new (41 days; range: 1–164) and retreatment TB patients (38 days; range: 8–90) (Table 2a) but was significantly (p = 0.029) longer for minors (52 days; range: 5–163) compared to adults (36 days; range: 1–164) (Table 2b). Total pathway delay was seen in 23 (36%) patients. Proportions of new (35%, n = 17/49) and retreatment TB patients (40%, n = 6/15) with total pathway delay were similar (Table 2a) but a higher proportion of minors (47%, n = 9/19) compared to adults (31%, n = 14/45) showed delay (Table 2b). Outliers for total pathway delay constituted 43% (n = 10/23) of patients with delay. Proportion of outliers among new TB patients with delay (41%, n = 7/17) was less compared to those among retreatment patients with delay (50%, n = 3/6), while the proportion of outliers among minors with delay (56%, n = 5/9) was more than those among adults with delay (36%, n = 5/14).
| New (n = 49) | Retreatment (n = 15) | Entire cohort (n = 64) | |
|---|---|---|---|
| Total patient pathway | |||
| Duration < 35 days | 32 (65%) | 9 (60%) | 41 (64%) |
| Duration ≥ 35 days | 17 (35%) | 6 (40%) | 23 (36%) |
| Mean in days (range) | 41 (1–164) | 38 (8–90) | 40 (1–164) |
| Median in days | 27 | 26 | 27 |
| First care seeking | |||
| Duration < 15 days | 34 (69%) | 10 (67%) | 44 (69%) |
| Duration ≥ 15 days | 15 (31%) | 5 (33%) | 20 (31%) |
| Mean in days (range) | 15 (0–122) | 16 (0–61) | 15 (0–122) |
| Median in days | 7 | 13 | 9 |
| Diagnostic delay | |||
| Duration < 15 days | 31 (63%) | 9 (60%) | 40 (62%) |
| Duration ≥ 15 days | 18 (37%) | 6 (40%) | 24 (38%) |
| Mean in days (range) | 24 (0–131) | 22 (1–71) | 23 (0 – 131) |
| Median in days | 9 | 8 | 9 |
| Treatment initiation | |||
| Duration < 7 days | 44 (90%) | 15 (100%) | 59 (92%) |
| Duration > 7 days | 5 (10%) | 0 | 5 (8%) |
| Mean in days (range) | 2 (0–30) | 0 (0–5) | 2 (0–30) |
| Median in days | 0 | 0 | 0 |
Durations and delays among new and retreatment patients.
| Minor (n = 19) | Adult (n = 45) | Entire cohort (n = 64) | |
|---|---|---|---|
| Total patient pathway | |||
| Duration < 35 days | 10 (53%) | 31 (69%) | 41 (64%) |
| Duration ≥ 35 days | 9 (47%) | 14 (31%) | 23 (36%) |
| Mean in days (range) | 52 (5–163) | 36 (1–164) | 40 (1–164) |
| Median in days | 31 | 27 | 27 |
| First care seeking | |||
| Duration < 15 days | 14 (74%) | 30 (67%) | 44 (69%) |
| Duration ≥ 15 days | 5 (26%) | 15 (33%) | 20 (31%) |
| Mean in days (range) | 11 (2–31) | 17 (0–122) | 15 (0–122) |
| Median in days | 9 | 9 | 9 |
| Diagnostic delay | |||
| Duration < 15 days | 10 (53%) | 30 (67%) | 40 (62%) |
| Duration ≥ 15 days | 9 (47%) | 15 (33%) | 24 (38%) |
| Mean in days (range) | 38 (0–131) | 17 (1–106) | 23 (0–131) |
| Median in days | 8 | 9 | 9 |
| Treatment initiation | |||
| Duration < 7 days | 18 (95%) | 41 (91%) | 59 (92%) |
| Duration > 7 days | 1 (5%) | 4 (9%) | 5 (8%) |
| Mean in days (range) | 2 (0–30) | 2 (0–30) | 2 (0–30) |
| Median in days | 0 | 0 | 0 |
Durations and delays among minor and adult patients.
No associations between gender, education, occupation, type of symptoms and total patient pathway were noted.
3.5. First care seeking: durations and delays
The mean duration was 15 days for the entire cohort. No difference in duration was observed between new (15 days; range: 0–122) and retreatment TB patients (16 days; range: 0–61). Mean comparisons showed minors accessing care slightly faster (11 days; range: 2–31) than adults (17 days; range: 0–122). First care seeking delay was seen in 20 (31%) patients. No difference was seen in the proportion of new (31%, n = 15/49) and retreatment (33%, n = 5/15) patients with delay (Table 2a), but the proportion of adults showing delay (33%, n = 15/45) was more than minors (26%, n = 5/19) (Table 2b). Outliers for first care seeking delay constituted 50% of total TB patients with delay (n = 10/20). The proportion of outliers was more among new TB patients with delay (53%, n = 8/15) as compared to retreatment patients (40%, n = 2/5) (Fig. 2). In contrast to outliers for total pathway delay, the proportion of outliers for first care seeking delay was more among adults (53%, n = 8/15) as compared to minors (40%, n = 2/5).
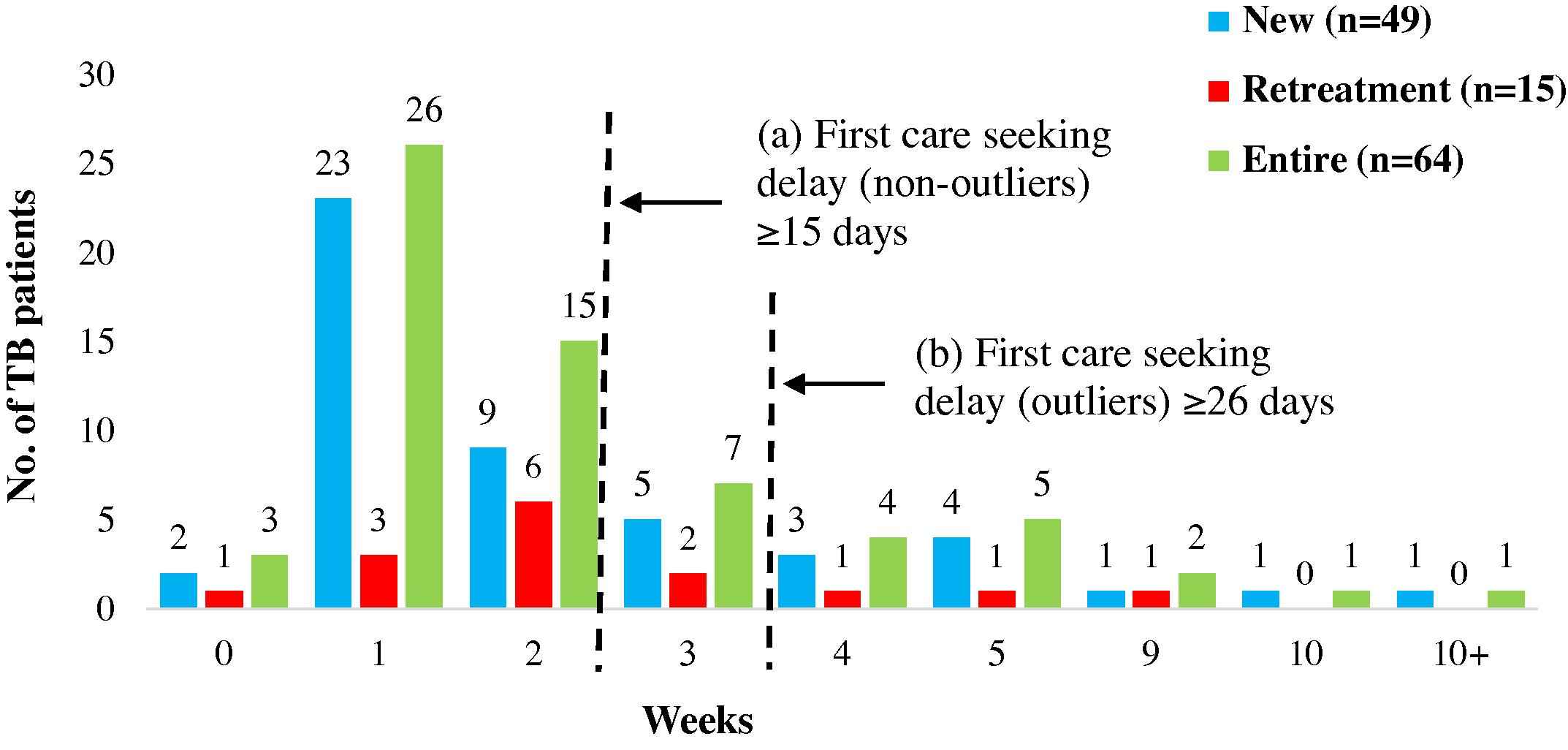
First Care Seeking: Durations and Delays. The figure represents the time taken from onset of symptoms suggestive of TB to first contact with any health care provider. The serrated lines depict the durations that reflect (a) delay and (b) outliers.
No significant associations between gender, education, occupation and first care seeking were noted.
Outliers were patients between the ages 35 and 54 years, illiterate, housewives, reporting cough and fever among major symptoms.
The reasons for their delay in seeking care were varied: while some had not felt the symptoms were serious enough, others attributed them to pollution or allergies. Financial reasons were reported by some as the reason for delay in seeking care (Table 3).
| Patients showing delay | ||
|---|---|---|
| First care seeking delay | Non-outliers 15–25 days (n = 10) |
Outliers ≥ 26 days (n = 10) |
| Did not deem symptoms serious enough | 3 | 3 |
| Attributed symptoms to other causes (allergy, weather, pollution, common cough, weakness, addictions) | 10 | 5 |
| Financial constraints | 1 | 4 |
| Tried home remedies | 1 | 0 |
| Family-related issues | 0 | 1 |
| Diagnostic delay | Non-outliers 15–39 days (n = 12) |
Outliers ≥ 40 days (n = 12) |
| Patient-related factors | ||
| provider shopping | 11 | 12 |
| delay in approaching provider after leaving previous provider | 8 | 10 |
| refusal to get tests done | 2 | 0 |
| Provider-related factors | ||
| advising symptomatic treatment for long duration | 7 | 7 |
| delay in advising TB-relevant tests | 3 | 4 |
| wrong diagnosis | 1 | 2 |
| Referral | 0 | 1 |
| Treatment initiation delay | Non-outliers 8–14 days (n = 3) |
Outliers ≥ 15 days (n = 2) |
| Patient-related factors | ||
| provider shopping | 0 | 1 |
| Lack of money | 1 | 1 |
| delay in approaching provider after leaving previous provider | 0 | 1 |
| Provider-related factors | ||
| Referral by diagnosing provider to another provider for initiation of treatment | 2 | 0 |
| long symptomatic treatment (despite TB diagnosis by previous provider) | 1 | 0 |
Some patients had no response or more than one response.
Reasons for delay.†
3.6. Diagnosis: durations and delays
Mean diagnostic duration was the longest (23 days; range: 0–131), contributing 58% to the total pathway and attributing to most of the delay in patient pathways. Diagnostic delay was seen in 24 (38%) patients. There was no difference in diagnostic duration between new (24 days; range: 0–131) and retreatment TB patients (22 days; range: 1–71). However a significantly (p = 0.000) longer diagnostic duration was seen in minors (38 days; range: 0–131) compared to adults (17 days; range: 0–106). As seen in first care seeking delay, a similar proportion of new (37%, n = 18/49) and retreatment (40%, n = 6/16) patients showed diagnostic delay however in contrast, a larger proportion of minors (47%, n = 9/19) showed diagnostic delay as compared to adults (33%, n = 15/45). Outliers for diagnostic delay constituted 50% of TB patients with delay (n = 12/24). As opposed to outliers for first care seeking, outliers for diagnostic delay constituted an equal proportion of new (50%; n = 9/18) and retreatment patients with delay (50%; n = 3/6) (Fig. 3), while the proportion of outliers among minors with delays (67%; n = 6/9) was more as compared to adults (40%; n = 6/15).
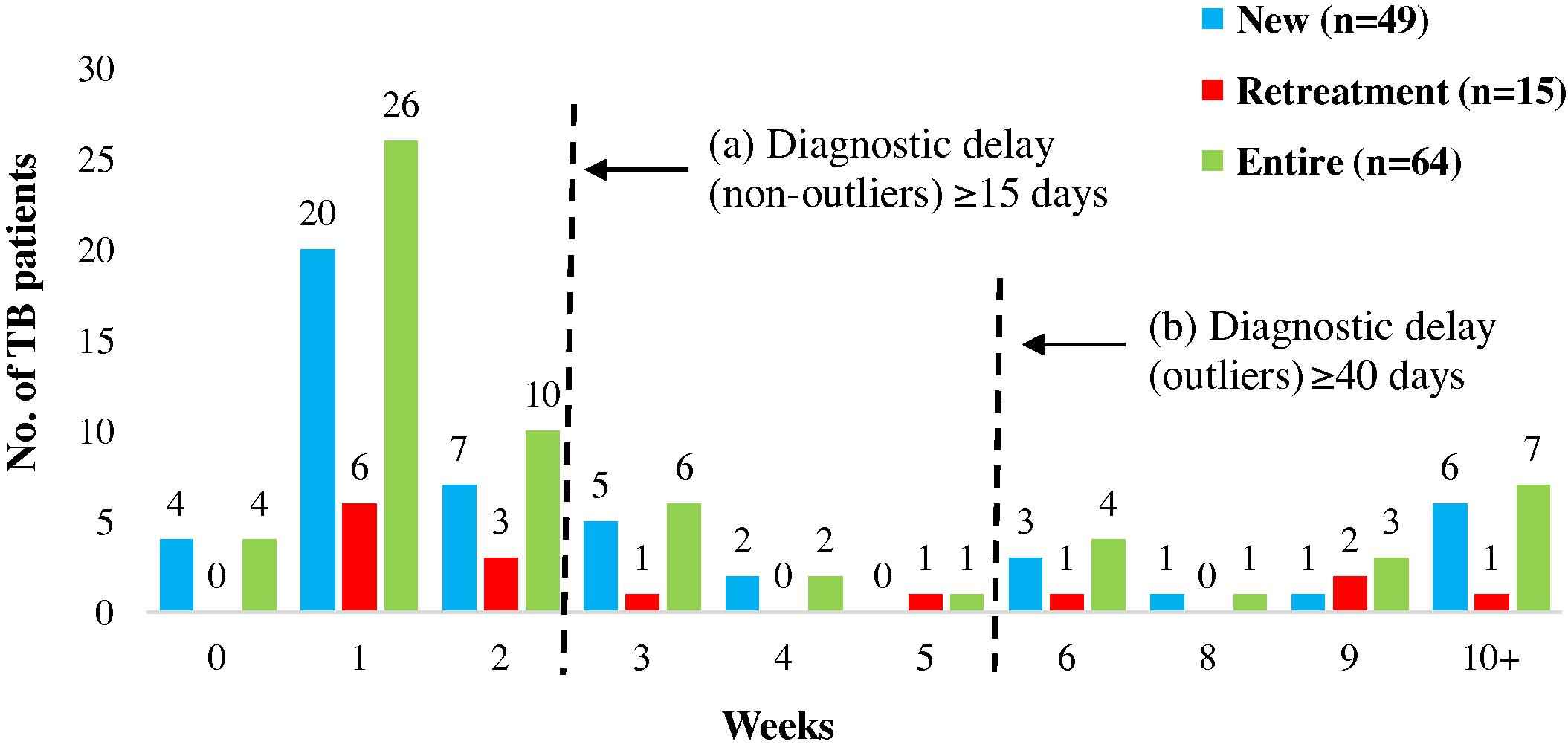
Diagnosis: durations and delays. The figure represents the time taken from first contact with any health care provider to receiving PTB diagnosis. The serrated lines depict the durations that reflect (a) delay and (b) outliers.
No associations were noted between gender, education, occupation, classification and diagnostic delay. Though not significant, 70% of patients (n = 28/40) with no diagnostic delay had been diagnosed in the private sector. Provider shopping was a key patient-related factor contributing to diagnostic delays. Predominant reasons for shopping were: no relief obtained from the care given by the provider, desire to seek treatment from Ayurvedic (alternative medicine) providers, distance of provider from the patient’s residence etc. Another major contributing factor was delay (≥7 days) in approaching the next provider after leaving one provider (Table 3).
A provider-related factor contributing to diagnostic delay was advising symptomatic treatment for long duration without advising TB-related tests. Investigations for extrapulmonary symptoms contributed to delays in two outliers.
3.7. Treatment initiation: durations and delays
Of the entire pathway, the duration for TB treatment initiation was the least. The mean treatment initiation duration for the entire cohort and new TB patients was 2 days (range: 0–30), while that for retreatment patients was none (range: 0–5) as most patients were initiated on TB treatment immediately after diagnosis (Table 2a). Five new patients (4 adults, 1 minor) (8%) experienced delay in treatment initiation. Two of these were outliers (Fig. 4), a male child and an illiterate housewife, both of who were diagnosed and initiated on ATT in the private sector. The reasons for delay were provider shopping for confirmation of diagnosis for the former and patient delay in approaching the provider after a spell of symptomatic treatment for the latter.
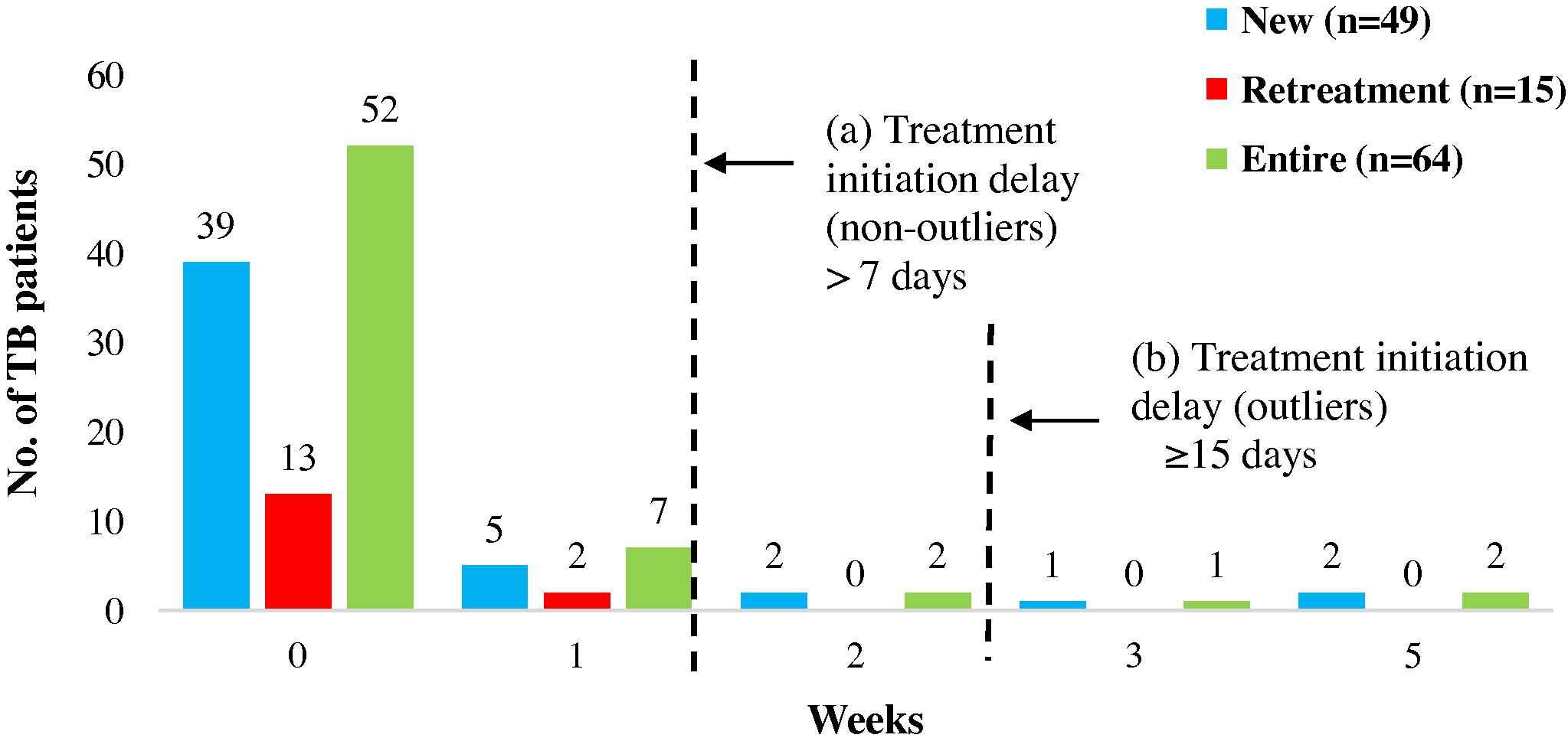
Treatment initiation: durations and delays. The figure represents the time taken from diagnosis of PTB to the initiation of anti-TB treatment. The serrated lines depict the durations that reflect (a) delay and (b) outliers.
Associations between age, gender, education, occupation, classification and treatment initiation were not observed.
Provider-related factors responsible for treatment initiation delays were primarily due to active referrals by the diagnosing provider to another provider for treatment initiation since treatment costs were unaffordable for the patients.
3.8. Provider switching
The first point of care for most patients (94%, n = 60/64) was the private sector (Fig. 5A).
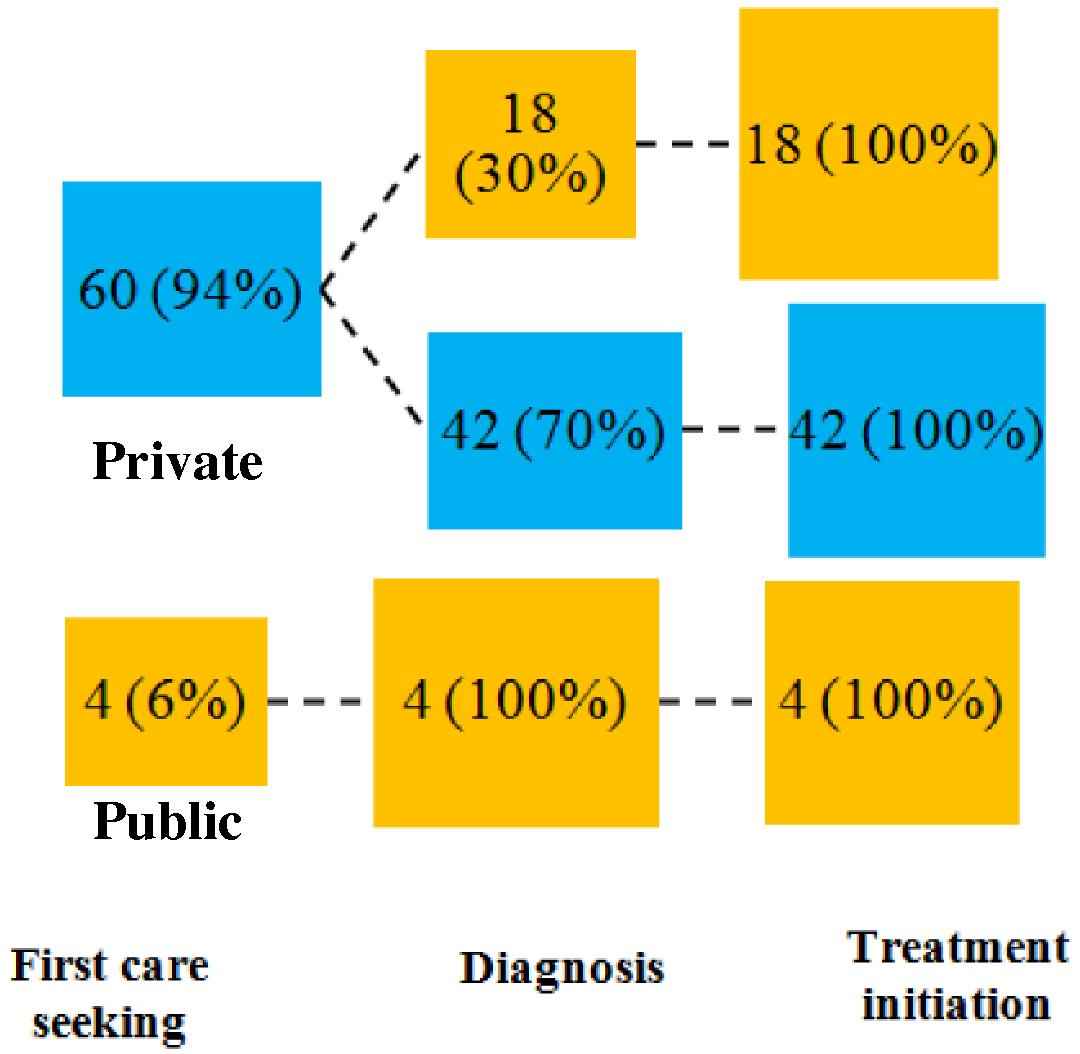
Provider switching between public and private sector at different stages of the pathway. The figure depicts the patient behaviour in seeking TB care at different stages of the pathway and preference for accessing the public or private sector.
The preference for this sector persisted in the downstream stages of the pathway - TB diagnosis and ATT initiation; 70% of those whose first point of care was the private sector were diagnosed in the private sector (n = 42/60) and all these patients were initiated on treatment in the private sector. On an average, patients visited three providers until initiation of TB treatment.
It is interesting to note that four patients who approached and continued with the public sector until treatment initiation, had shorter mean total pathway duration compared to those accessing the private sector (23 days vs. 41 days) (p = 0.079). Provider switching from private to public sector resulted in the addition of a single day to the duration compared to those who remained within the private sector. (1 day vs. 2 days) (Fig. 5A).
Fig. 5B depicts 93% of patients approached allopaths for first care seeking while 7% approached non-allopaths. As opposed to sector-based switching, those who accessed allopaths initially did not switch to non allopaths: diagnosis and treatment initiation in Patna was effected only by allopaths (100%).
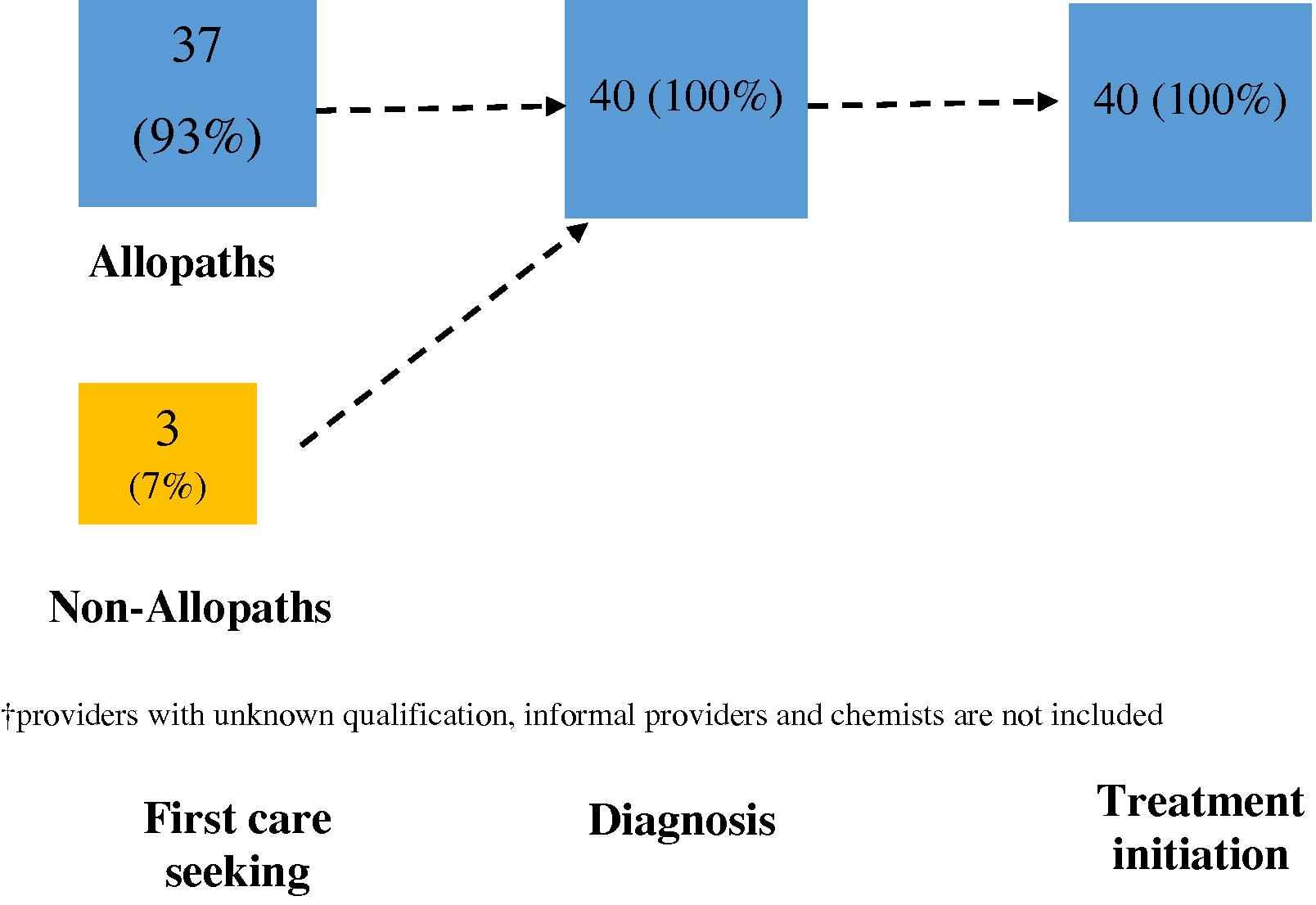
Provider switching between allopaths and non-allopaths at different stages of the pathway†. The figure depicts the patient behaviour in seeking TB care at different stages of the pathway and preference for seeking care from allopaths or non-allopaths.
3.9. Analysis on basis of recall
Patients were analyzed based on recall periods of less than six months and beyond from symptom onset to interview date. Forty-five percent patients (n = 29/64) were interviewed within six months of onset of symptoms and 55% (n = 35/64) beyond six months.
The difference in total pathway duration between patients with recall period of more than six months and patients with recall period of six months or less was merely 4 days (42 days vs. 38 days) (p ≥ 0.05). However patients with longer recall period had significantly longer first care seeking duration (18 days vs. 11 days) (p = 0.026). Both groups showed similar diagnostic (23 days vs. 24 days) and treatment initiation duration (1 day vs. 3 days).
For new patients with longer recall periods, first care seeking duration was significantly longer in comparison to patients with shorter recall period. (19 days vs. 11 days) (p = 0.021). Retreatment patients in both groups had similar duration for the same part of the pathway (16 days vs. 15 days).
4. Discussion
The study describes the pathway to care for pulmonary TB patients in the city of Patna in Bihar state considered as an economically backward one amongst its peers [18]. Since there is a relative dearth of information from Patna, the findings are discussed in comparison to a similar survey in Mumbai undertaken concurrently [6].
Compared to Mumbai, Patna exhibited striking contrasts in several aspects. Firstly the subjects interviewed were the indigenous population of Patna and not interstate/intrastate migrants that formed the sample in Mumbai [6]. Based on the interviews, it was observed that patients in Patna were relatively more aware of their healthcare providers and their qualifications in their vicinity and had somewhat better treatment literacy for their condition despite comparable education status in both cities (data not shown).
Almost 30% of the interviewees comprised of minors where first care seeking delays were lower than adults. Concern for minors coupled with increased awareness of the disease maybe another reason for faster first care seeking among parents for their children. However, despite seeking care early, the diagnostic duration for minors was more than adults and there was a large representation of minors as outliers for diagnostic delay because of overlay of other common respiratory ailments confusing the diagnosis, poor expectoration of sputum and the lack of facilities like nebulization and gastric lavages and culture [19]. Rapid access but an extended diagnostic delay was also highlighted in childhood TB patients in Delhi [20].
The attraction of the private sector for TB patients in this study is undoubtedly striking. While this was true even in Mumbai at the first point of care [6,21], there was persistent adherence in Patna to the private sector and indeed even to the same provider throughout the pathway, with relatively reduced shopping (4 providers in Patna vs. 7 providers in Mumbai). However it was still a major cause of delay due to long intervals between sequential providers. This may reflect, partially, a lack of confidence in the public sector offering care for TB, where many doctors are perceived to undertake private practice concurrently [22].
The overwhelming proportion of patients accessing private allopathic providers and chemists was another singular finding. However it needs to be ascertained whether the claim of being a genuine allopathic doctor is true. With its over-commercialized education system where qualifications are purchasable, it is likely that many who are identified as allopathic practitioners’ maybe non-degree allopathic [23] or even AYUSH practitioners who follow biomedical models [24]. Whilst non-allopathic doctors of all hues in Mumbai [6] maybe willing to refer but rarely to treat, the same hue in Patna may have confidence in undertaking even treatment due to weak regulation.
The fact that despite a wide range of doctors treating TB, and predictable antibiotic misuse, the representation of Drug-resistant TB (DR-TB) patients in the cohort was absent in contrast to the 28% of DR-TB patients picked up in the Mumbai cohort. The virtual absence of both phenotypic (MGIT/culture) and genotypic (GeneXpert) technology for detection of DR patients in Patna (World Health Partners, personal communication) maybe compounding provider inefficiency. Patna, like several other cities in India with a similar health care scenario, needs to be aware of this problem and abandon a false sense of security. The end-line study to measure the effect of the PPIA intervention may throw more light on the true extent of DR-TB disease in Patna.
Like in Mumbai [6], retreatment patients showed almost an equal extent of delay as new patients in first care seeking, diagnosis and total pathway. This highlights the need for counseling of TB patients at the time of release from treatment, so that timely care seeking is effected at the time of relapse.
Patna shares with Mumbai also the bias of extended recall during the course of the interviews. However analysis of the patients based on the time from onset of symptoms to interview showed that both groups of patients with a longer and shorter recall period had comparable delays.
Lastly, the shorter pathway may also be a tendency of patients to underestimate durations despite the fact that every effort was made to triangulate responses of duration with key events. Behavioural ethnographic studies on care seeking in heterogeneous patients may help in fine-tuning the veracity of pathway studies for chronic conditions [24,25].
5. Conclusion
Given this scenario of health care providers, one would expect a longer care pathway for TB patients, compared to their counterparts in Mumbai and several low and middle income countries. However, this was not the case: the total pathway duration was lesser by over three weeks in Patna compared to Mumbai (40 vs. 65 days) [6] and other cities in India [26]. A systematic review (2014) of Indian cities shows shorter total pathways associated with access to public sector at first point of care. Patna seems to be unique as it shows shorter total pathway with predominant care seeking from the private sector [26]. This is probably not due to provider efficiency but more due to active seeking of diagnosis and care in a perceived milieu of minimal stigma, which is more strongly entrenched in situations like Mumbai, where a high prevalence of migrants lends to perspectives of “outsiders” generally generated by the media [27].
In the absence of data on TB management practices from private practitioners catering to the needs of the population studied, one can only surmise from previous findings [28] that a combination of public health services perceived to be weak by both people and the private health providers, poor availability of advanced tests for TB diagnosis, and questionable quality of TB diagnosis and treatment offered by all or most private providers, may be at play in Patna.
Eventually, however, the quality of care provided and measured [29] and the ability and willingness of vulnerable patients to adhere to the care pathways will impinge on favorable outcomes for the patient. A rapid assessment scorecard to identify areas of vulnerable populations at risk for TB maybe an important step for concentrating control efforts for optimal outcomes [30].
Authors contributions
NM, YD were involved in the conception and design. NM, EL, SS, SR, YD were involved in data analysis/ interpretation. EL, NM were involved in drafting of paper and revising the draft. SS, SR, YD were involved in critical analysis of the paper. All the authors have approved the final version of the article submitted.
Competing interests
None declared.
Funding source
The Bill and Melinda Gates Foundation Grant number OPP1091874 through Sambodhi Research & Communications Pvt. Ltd. to NM. The funders had no role in study design, analysis, decision to publish, or preparation of manuscript.
Acknowledgements
The study was funded by the Bill and Melinda Gates Foundation through Sambodhi Research and Communication Pvt. Ltd. The authors are thankful to Sambodhi Research and Communication Pvt. Ltd. (Mr. Kultar Singh and Mr. Paresh Kumar) team for the initial household survey in Patna. We are grateful to Ms. Akshaya Patil for her supervisory role in the study, along with all our field researchers from the Foundation for Medical Research (FMR) based in Patna for data collection in the field.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jegh.2017.08.001.
References
Cite this article
TY - JOUR AU - Nerges Mistry AU - Eunice Lobo AU - Shimoni Shah AU - Sheela Rangan AU - Yatin Dholakia PY - 2017 DA - 2017/08/10 TI - Pulmonary tuberculosis in Patna, India: Durations, delays, and health care seeking behaviour among patients identified through household surveys JO - Journal of Epidemiology and Global Health SP - 241 EP - 248 VL - 7 IS - 4 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2017.08.001 DO - 10.1016/j.jegh.2017.08.001 ID - Mistry2017 ER -
