Epidemiology, clinical manifestations, and molecular typing of salmonella typhi isolated from patients with typhoid fever in Lebanon
Both authors contributed equally to the manuscript.
- DOI
- 10.1016/j.jegh.2014.07.003How to use a DOI?
- Keywords
- Salmonella typhi; Typhoid fever; Enteric fever; Food borne diseases; Molecular typing; Middle east
- Abstract
The objective of this study was to examine the epidemiology and the clinical manifestations of typhoid fever as well as the susceptibility and strain relatedness of Salmonella typhi isolates in Lebanon from 2006 to 2007. A total of 120 patients with typhoid fever were initially identified from various areas of the country based on positive culture results for S. typhi from blood, urine, stools, bone marrow and/or positive serology. Clinical, microbiological and molecular analysis was performed on cases with complete data available. These results indicated that drinking water was an unlikely mode of transmission of the infection. Despite increasing reports of antimicrobial resistance among S. typhi isolates, the vast majority of these isolates were susceptible to various antibiotic agents, including ampicillin, cephalosporins, quinolones, and trimethoprim/sulfamethoxazole. Molecular analysis of the isolates revealed a predominance of one single genotype with no variation in distribution across the geographical regions.
- Copyright
- © 2014 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Typhoid fever is a systemic infection caused by the enteric gram-negative bacterium Salmonella enterica serovar typhi (S. typhi) [1,2]. This food and waterborne disease is strongly correlated with poor hygiene as well as overpopulated areas with poor sanitation [1,3]. Typhoid fever continues to be a serious global public health problem and is a major cause of morbidity and mortality in the developing world. High areas of endemicity include South-central Asia, Southeast Asia, and Southern Africa.
According to the World Health Organization, it is estimated that the yearly incidence of typhoid fever exceeds 21 million cases with over 200,000 deaths [4]. In the United States, 200–300 new cases are reported annually, most of which occur in travelers returning from endemic countries [5]. Epidemiological methods to determine clonal relatedness have been increasingly used in various settings. Pulsed-field gel electrophoresis (PFGE) is a well-established and highly effective epidemiological tool for the molecular analysis of large fragments generated by restriction endonuclease digestion of genomic DNA. It is used to subtype S. typhi for surveillance purposes, to identify and investigate outbreaks, as well as to characterize endemic strains. The use of PFGE with endonuclease XbaI is now widely recognized as a sensitive means of fingerprinting Salmonella serovars, and it has recently become a reference method [6].
Although historically initiated in 1957, surveillance for communicable diseases in Lebanon was interrupted during the civil war from 1975 to 1994 and was only re-initiated in 1995 [7]. The local epidemiology of typhoid fever has not been well established, hence the rationale behind this study. The objective was to describe the clinical manifestations and risk factors for typhoid fever in this patient population. Antimicrobial susceptibility and molecular analysis to determine the strain relatedness of the collected S. typhi isolates were also performed, and the results were correlated with the clinical data.
2. Methods
2.1. Identification of subjects and data collection
This was an observational study conducted at 14 hospitals in Lebanon. Cases of typhoid fever were identified between October 2006 and January 2008 based on positive culture results for S. typhi from blood, urine, stools, bone marrow and/or positive serology obtained from microbiology laboratories. Only one culture per patient was included. Patient-specific clinical and laboratory data were collected retrospectively from patients’ medical records. Information was collected on patient demographics, area of residence, source of water supply, socioeconomic status and living conditions, clinical signs and symptoms upon presentation, and laboratory, radiological and pathologic findings, in addition to the treatment regimen and final outcome. Data entry and statistical analysis were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Because of the non-comparative nature of the study, only descriptive statistics were performed.
2.2. Microbiological analysis
The detection, identification and susceptibility testing of S. typhi from clinical specimens were done at the Clinical Microbiology Laboratory of the Department of Pathology and Laboratory Medicine. This Laboratory has been accredited by the College of American Pathologists (CAP) since 2004. Briefly, the recovery of S. typhi isolates from blood was based on the BactAlert system (Bio Merieux, Marcy-l’Etoile, Lyon, France), and sub-culturing positive bottles on blood, chocolate, and Mac Conkey agar media (Becton Dickinson Microbiology Systems, NJ, USA). Recovery from stool specimens was based on the direct inoculation of specimen on selective media, XLD (Xylose-Lysine-Deoxycholate) agar, and Selenite broth for the enrichment of Salmonella and Shigella. After 12–18 h, the Selenite broth was subcultured on SS (Salmonella-Shigella) agar. All suspected colonies, especially those with positive H2S reaction, were identified based on biochemical sugar reactions, API E system (Bio Merieux, Marcy-l’Etoile, Lyon, France) and by serotyping with specific anti-Salmonella anti-sera (Denka Seiken Co., Coventry, UK). Isolates were stored in Brucella-Glycerin broth storage medium (BD). Antimicrobial susceptibility testing was done by the disk-diffusion (Kirby-Bauer) method using suspensions of 0.5 MacFarland from fresh bacterial cultures on Mueller-Hinton agar (BD) according to the guidelines of the Clinical Laboratory Standards Institute (CLSI, 2009) [8]. E. coli ATTCC # 25,9922 was used as a quality control reference strain. The following antimicrobial disks (BD) were used: ampicillin (disk content 10 μg), SXT, ceftazidime (30 μg), cefotaxime (30 μg), ciprofloxacin (5 μg), norfloxacin (10 μg), and ceftriaxone (30 μg). In addition, nalidixic acid disk (30 μg) was used to screen for fluoroquinolone resistance. If an isolate showed resistance to nalidixic acid, a note in the report was added: “since isolate is resistant to nalidixic acid, treatment with fluoroquinolones might be compromised.” The performance and interpretation of this procedure and results are based on the 2009 CLSI guidelines.
2.3. Molecular analysis
All S. typhi isolates underwent Pulsed-Field Gel Electrophoresis (PFGE) to study their genotypic patterns. PFGE was carried out according to PulseNet protocol (PulseNet International) using the restriction enzyme XbaI (Fermentas Life Sciences, Canada), with Salmonella Braenderup H9812 as a reference strain. Electrophoresis was performed using CHEF MAPPER (Bio Rad Laboratories GMBH, Munchen, Germany). The gels were stained with ethidium bromide (AMERCO, Ohio, USA) and photographed under UV light illumination by Gel Doc (Bio Rad Laboratories GMBH, Munchen, Germany). The images were analyzed with BIONUMERICS 5.1 (Applied Maths, Saint-Martens-Latem, Belgium). 2.5% tolerance and optimization criteria were imposed for the analysis, according to Dice’s criterion. The dendrogram was constructed with UPGMA (Unweighted Pair Group Method using Arithmetic Averages).
3. Results
3.1. Clinical data
A total of 120 patients with typhoid fever were identified over the course of the study period from various geographical locations in the country (Fig. 1). Clinical data were complete and available for 69 patients (33 males and 36 females); the remaining 51 patients with missing clinical data were excluded from the clinical analysis. The mean age of enrolled patients was 23 years (median 19 years; range 1–82 years). The majority of patients were residents of urban areas, with 40% residing in the capital Beirut (Figs. 2A and B). The average number of household members was 5 (range 1–11), and the highest attained level of education was reported as elementary for 35.7% of patients and college degree for 28.6% of patients. A sizable proportion of patients relied on bottled water as the source of drinking water at home (50.9%), followed by drinking water from commercial providers (31.6%) and municipal water system (17.5%). Additionally, the majority of homes were connected to the municipal sewage system (68%), while others were connected to cesspools (32%).
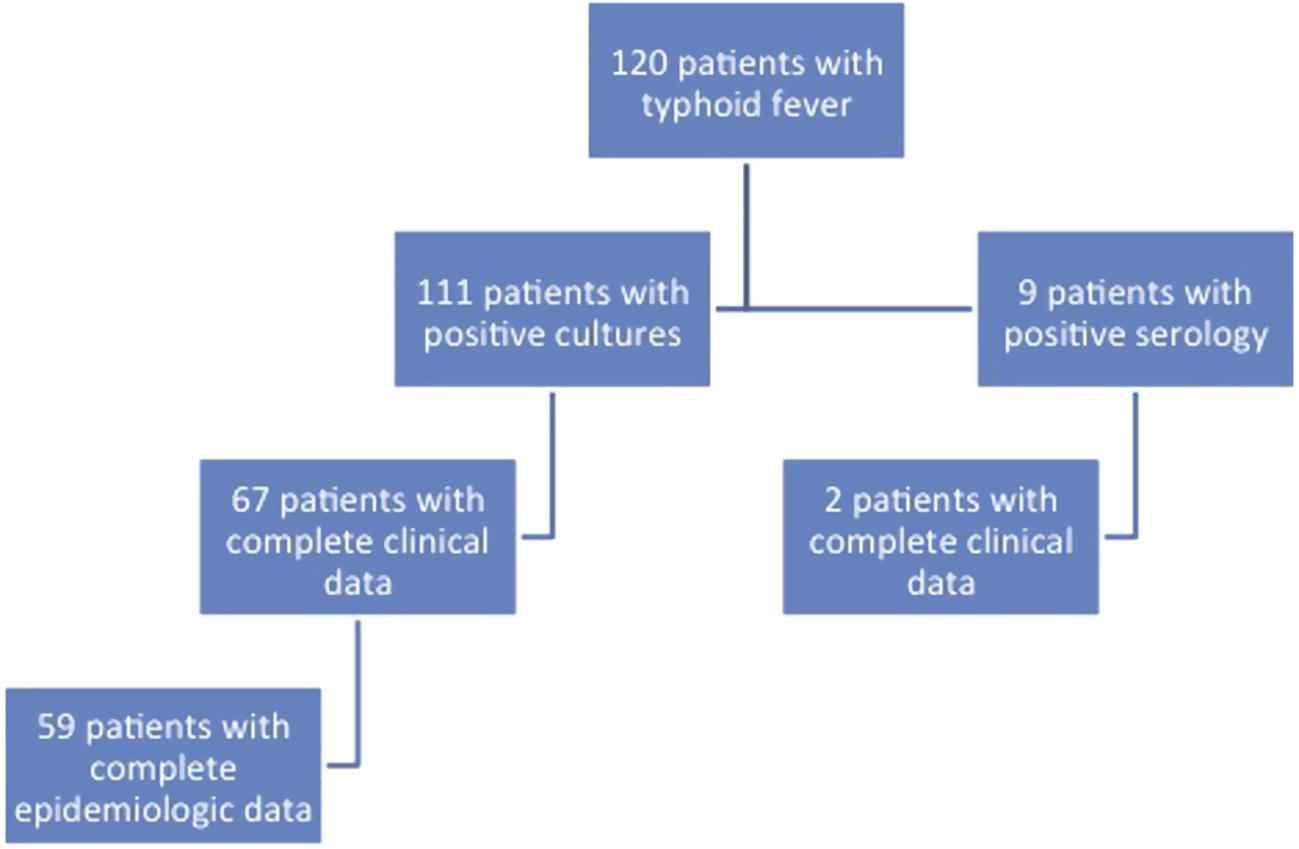
Flowchart of the distribution of patients included in the study.
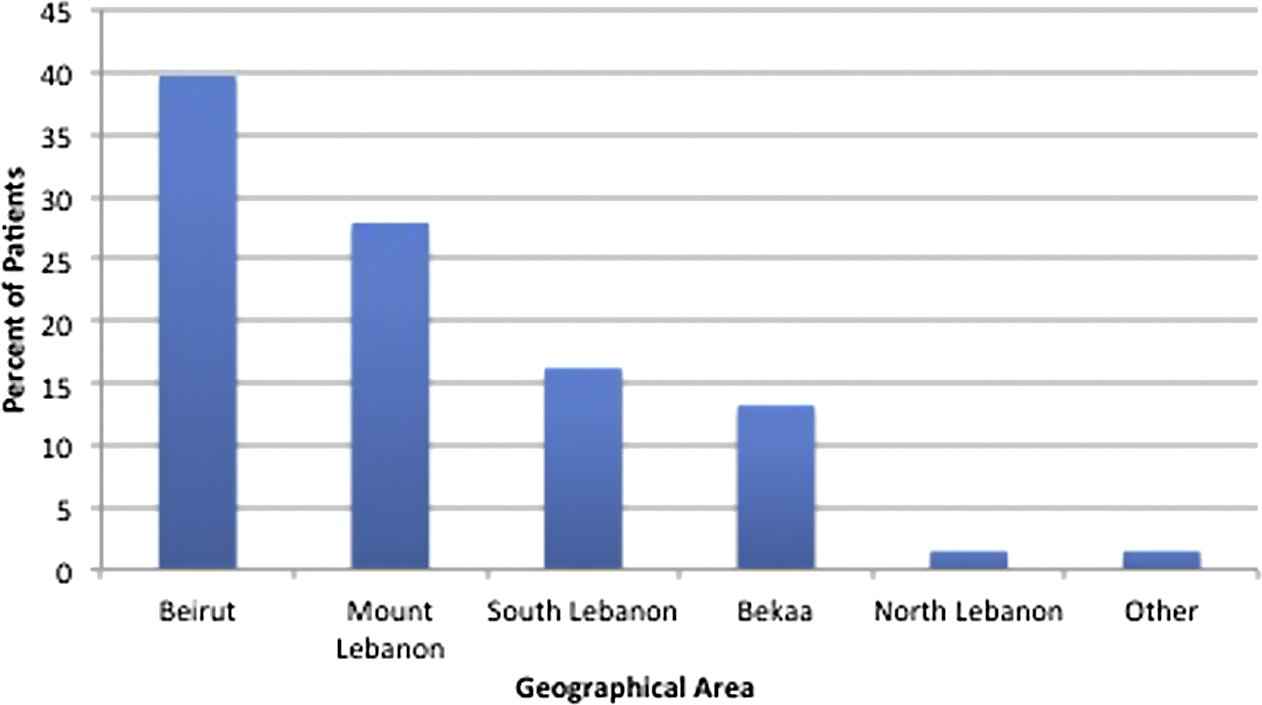
Geographic distribution by regions of residence in Lebanon of patients with confirmed typhoid fever.
Map of Lebanon showing various geographic regions (Courtesy of Google search: http://www.sodetel.net.lb/common/flash/map.swf).
The clinical characteristics of patients are summarized in Table 1. Only a small fraction of patients had serious co-morbid conditions (11.7%). Four patients (6.2%) had a previous episode of typhoid fever within the 6 months preceding the current episode. In addition, 7 patients (12.3%) reported a similar illness in the household concurrent with their illness. The most common presenting symptoms were fever (100%), headache (56.5%), abdominal pain (46.4%), diarrhea (40.6%) and vomiting (39.1%). Two patients who were sisters presented with genital ulcerations.
| Patient Characteristics | Value N (%) |
|---|---|
| Comorbidities | |
| Diabetes mellitus | 3 (4.4) |
| Congestive heart failure | 3 (4.4) |
| Immunosuppressive therapy | 2 (2.9) |
| Signs and symptoms | |
| Fever | 69 (100) |
| Headache | 39 (56.5) |
| Abdominal pain | 32 (46.4) |
| Diarrhea | 28 (40.6) |
| Vomiting | 27 (39.1) |
| Constipation | 8 (11.6) |
| Oral lesions | 7 (10.1) |
| Hepatomegaly and/or splenomegaly | 6 (8.7) |
| Genital lesions | 2 (2.9) |
| Need for hospitalization | 64 (92.8) |
| Similar illness in the household | 7 (12.3) |
| Typhoid fever within past 6 months | 4 (6.2) |
| Duration of fever, mean (range) | 8 (2–30) |
| Length of hospital stay, mean (range) | 6 (1–13) |
| Survival | 69 (100) |
Clinical characteristics of patients with typhoid fever in Lebanon between October 2006 and January 2008 (N = 69).
Of the 120 patients included in the study initially, 111 were diagnosed by blood or stool culture, and 9 were diagnosed by serological testing. Of the 69 patients included in the clinical analysis, the diagnosis was confirmed through blood cultures in 66 patients, serology in 2 patients, and stool culture in 1 patient in the setting of a compatible clinical illness. Third generation cephalosporins were the most commonly used agents for the treatment of typhoid fever (n = 48; 69.5%), followed by quinolones (n = 20; 29.5%). The mean duration of antibiotic treatment was 14 ± 2.8 days (range 5–21 days). All patients recovered from the illness without any serious complications.
3.2. Microbiological data
Susceptibility testing of the recovered isolates revealed resistance to ampicillin and trimethoprim/sulfamethoxazole in only 1 isolate, which maintained susceptibility against third generation cephalosporins and quinolones. All other isolates showed uniform susceptibility to ampicillin, third generation cephalosporins, quinolones (including nalidixic acid), and trimethoprim/sulfamethoxazole.
3.3. Molecular data
Molecular analysis and PFGE were completed on the 111 isolates received from patients. The dendrogram is shown in Fig. 3. Association with geographical area was confirmed by studies on 59 cases for which genotypic and epidemiological data were available. The most commonly identified cluster was cluster III. It accounted for a total of 49 isolates. The percent of isolates from various regions belonging to cluster III is distributed as follows: 62.5% from Bekaa, 47.0% from Mount Lebanon, 41.6% from Beirut, and 12.5% from South Lebanon. None of the isolates from North Lebanon belonged to cluster III. All other clusters were distributed evenly among all regions in Lebanon.
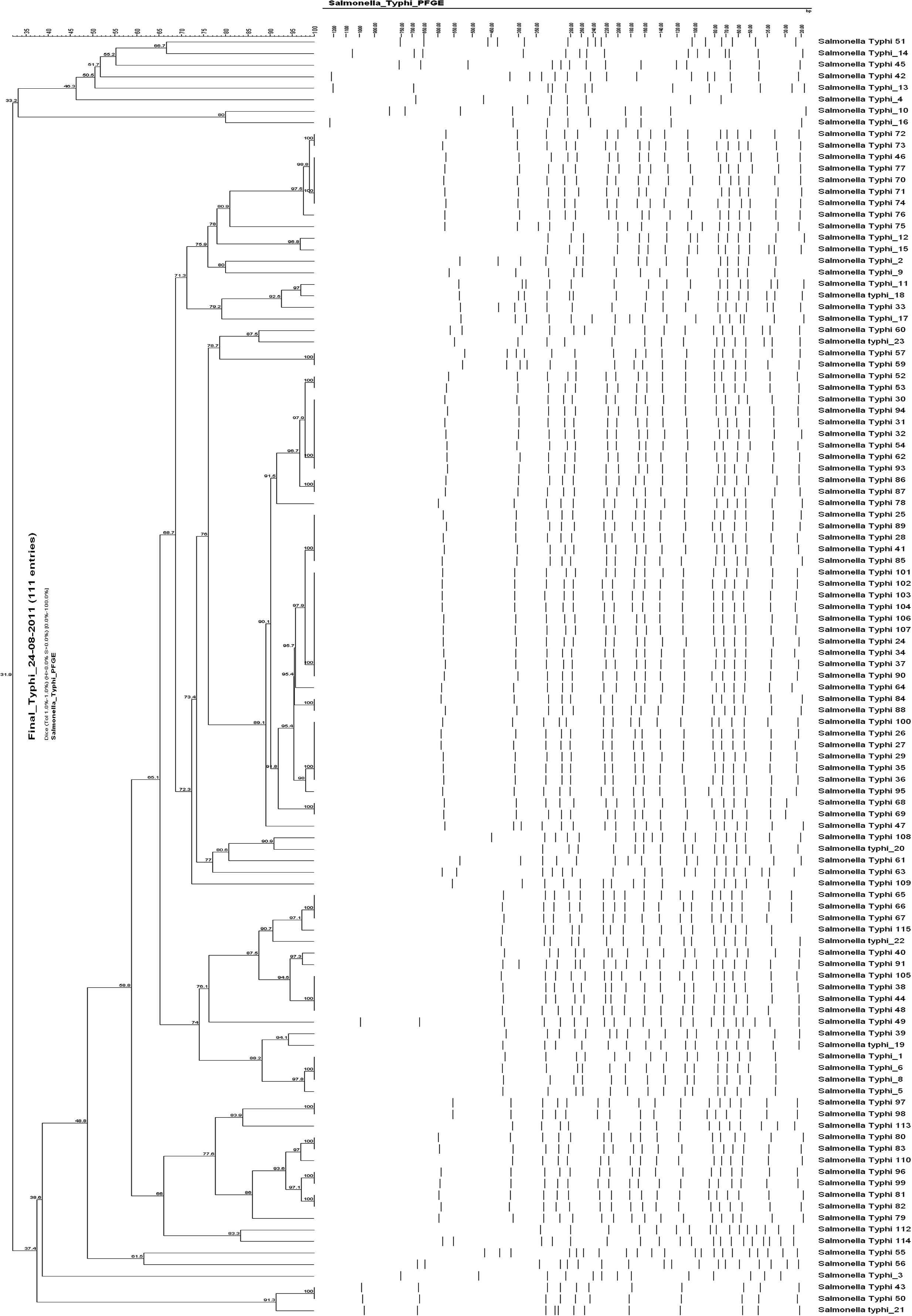
Dendrogram for 111 S. typhi isolates.
4. Discussion
Typhoid fever continues to be a problem in Lebanon, especially during the summer months. In a recent epidemiological study, a total of 6,148 cases of typhoid fever were reported in Lebanon between 2000 and 2008, with an annual range from 461 to 891 cases, and with the highest incidence occurring from June to August [9]. Similar to this current cohort, most patients in the national study were children and young adults between the ages of 10 and 39 years. Except for the genital ulcers seen in the 2 sisters [10] and the common occurrence of diarrhea in up to 40% of patients, the clinical manifestations seen in this patient population were similar to what has been previously reported in the literature. These results showed that typhoid fever occurred mostly in younger patients living in crowded households. This finding is consistent with several epidemiological studies showing that low socioeconomic status and poor living conditions are risk factors for acquiring the infection [11]. Contaminated food and water supplies have been frequently identified as sources of typhoid fever outbreaks [12,13]. Since most patients in this study population consumed bottled water, it seems that drinking water is a less likely vehicle for the transmission of the pathogen, although contaminated bottled water has been reported from this country. Household water used for bathing, washing, and cooking, however, remains a possible explanation since it is widely contaminated.
There is growing evidence showing that multi-drug resistant S. typhi strains are increasing worldwide. A recent analysis of 41 cases of typhoid fever from Cambodia showed multi-drug resistance rates of 56% (against ampicillin, trimethoprim/sulfamethoxazole, and chloramphenicol) [14]. In another study from Canada [15], resistance of S. typhi isolates to nalidixic acid reached 80% while resistance to beta-lactams remained constant between 2002 and 2007. It should be kept in mind, however, that resistance to nalidixic acid predicts treatment failure with fluoroquinolones (despite in vitro susceptibility of the isolate to fluoroquinolones). Therefore, high resistance rates of S. typhi to nalidixic acid might preclude the use of quinolones in the empiric treatment of typhoid fever in Canada. On the other hand, other studies have indicated that while resistance to quinolones is now rampant among S. typhi, other conventional therapeutic options have regained susceptibility. For example, in the Indian subcontinent, susceptibility rates of 128 isolates of S. typhi to chloramphenicol, ampicillin, and trimethoprim exceeded 90% [16]. The results of this study confirm that in Lebanon, antimicrobial resistance among S. typhi isolates is still rare (Table 2). Therefore, ampicillin, cephalosporins, quinolones, or trimethoprim/sulfamethoxazole remain appropriate therapeutic choices in patients with typhoid fever who have acquired the infection locally. Recent data compiled from 2000 to 2011 at one large tertiary care teaching hospital in Beirut suggest that S. typhi has remained uniformly susceptible to third generation cephalosporins and quinolones. Susceptibility has ranged between 60% and 100% for ampicillin and between 43% and 100% for trimethoprim/sulfamethoxazole [17].
| 2006–07 | 2007–08 | 2008–09 | 2009–10 | 2010–11 | 2011–12 | 2012–13 | |
|---|---|---|---|---|---|---|---|
| Ampicillin | 93 | 92 | 100 | 71 | 84 | 60 | 100 |
| Cefotaxime | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ceftazidime | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ciprofloxacin | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Norfloxacin | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Trimeth/Sulfa | 93 | 100 | 100 | 43 | 84 | 60 | 100 |
Susceptibility data of S. typhi at the American university of Beirut Medical center between 1 July 2006 and 30 June 2013.
Molecular analysis revealed the predominance of one circulating genotype. However, there was no apparent geographical predilection. This can probably be accounted for by the high mobility of the Lebanese population, e.g., from place of residence to place of work. It should be noted, however, that due to the fact that a systematic sampling method was not followed, the isolates included in this study might not be representative of the country as a whole.
In conclusion, young healthy individuals living in crowded households are at risk of acquiring typhoid fever in Lebanon. Antimicrobial resistance among S. typhi isolates has so far been uncommon in this country.
5. Conflict of interest
No competing interest to declare.
6. Funding
This study was funded by the US Army Medical Research and Material Command under Contract/Grant/Cooperative Agreement No. W81XWH-08-1-0591. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army.
References
Cite this article
TY - JOUR AU - Souha S. Kanj AU - Zeina A. Kanafani AU - Marwa Shehab AU - Nisreen Sidani AU - Tania Baban AU - Kedak Baltajian AU - Ghenwa K. Dakdouki AU - Mohamad Zaatari AU - George F. Araj AU - Rima Hanna Wakim AU - Ghassan Dbaibo AU - Ghassan M. Matar PY - 2014 DA - 2014/09/10 TI - Epidemiology, clinical manifestations, and molecular typing of salmonella typhi isolated from patients with typhoid fever in Lebanon JO - Journal of Epidemiology and Global Health SP - 159 EP - 165 VL - 5 IS - 2 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2014.07.003 DO - 10.1016/j.jegh.2014.07.003 ID - Kanj2014 ER -
