A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims
- DOI
- 10.1016/j.jegh.2014.08.002How to use a DOI?
- Keywords
- Facemask; Hajj pilgrimage; Influenza; Middle East respiratory syndrome coronavirus; Viral respiratory tract infection
- Abstract
Background: Cost-effective interventions are needed to control the transmission of viral respiratory tract infections (RTIs) in mass gatherings. Facemasks are a promising preventive measure, however, previous studies on the efficacy of facemasks have been inconclusive. This study proposes a large-scale facemask trial during the Hajj pilgrimage in Saudi Arabia and presents this protocol to illustrate its feasibility and to promote both collaboration with other research groups and additional relevant studies.
Methods/design: A cluster-randomised controlled trial is being conducted to test the efficacy of standard facemasks in preventing symptomatic and proven viral RTIs among pilgrims during the Hajj season in Mina, Mecca, Saudi Arabia. The trial will compare the ‘supervised use of facemasks’ versus ‘standard measures’ among pilgrims over several Hajj seasons. Cluster-randomisation will be done by accommodation tents with a 1:1 ratio. For the intervention tents, free facemasks will be provided to be worn consistently for 7 days. Data on flu-like symptoms and mask use will be recorded in diaries. Nasal samples will be collected from symptomatic recruits and tested for nucleic acid of respiratory viruses. Data obtained from questionnaires, diaries and laboratory tests will be analysed to examine whether mask use significantly reduces the frequency of laboratory-confirmed respiratory viral infection and syndromic RTI as primary outcomes.
Conclusions: This trial will provide valuable evidence on the efficacy of standard facemask use in preventing viral respiratory tract infections at mass gatherings.
This study is registered at the Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN: ACTRN12613001018707 (http://www.anzctr.org.au).
- Copyright
- © 2014 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Viral respiratory tract infections (RTIs), including influenza, are a major burden to public health. During any inter-pandemic year, there are an estimated 1 billion cases of influenza worldwide with 3–5 million cases of severe illness and 300,000–500,000 deaths [1]. Fear of the global spread of serious respiratory disease persists in the light of past pandemics, such as the severe acute respiratory syndrome (SARS), the influenza A (H1N1) pdm09 virus, and the recent emergence of the Middle East respiratory syndrome coronavirus (MERS-CoV) [2]. Ever-increasing international travel intensifies the risk of the spread of emerging novel viruses, further intensifying the concern [3]. Mass gatherings such as the Hajj pilgrimage pose a particular risk for transmission of respiratory viruses [4,5].
Health authorities require cost-effective measures that prevent or limit the global transmission of respiratory diseases. Facemasks represent a simple and cheap supplement to the use of hand-washing, antivirals, and vaccination in the control of viral RTIs.
Results from previous studies examining the effectiveness of facemasks have been either conflicting or inconclusive [6–17]. A randomised controlled trial (RCT) in a household setting found that adherence to facemask use decreased the risk of influenza-like illness (ILI) [7]. Meta-analysis of data from 6 trials shows that wearing facemasks is protective against ILI, but did not show that facemasks are protective against laboratory-confirmed influenza, perhaps due to limitations of the studies (Fig. 1) [18]. Interestingly, one nested case–control study showed that intermittent facemask use was associated with a significantly greater risk of infection among healthcare workers at Hajj, suggesting perhaps that infectious material that settles on facemasks can become a source of direct hand transmission to the respiratory tract [19]. Standard facemasks may prevent: (i) acquisition of infection by droplet and/or aerosol spread, or conversely, (ii) transmission from infected subjects [20,21].
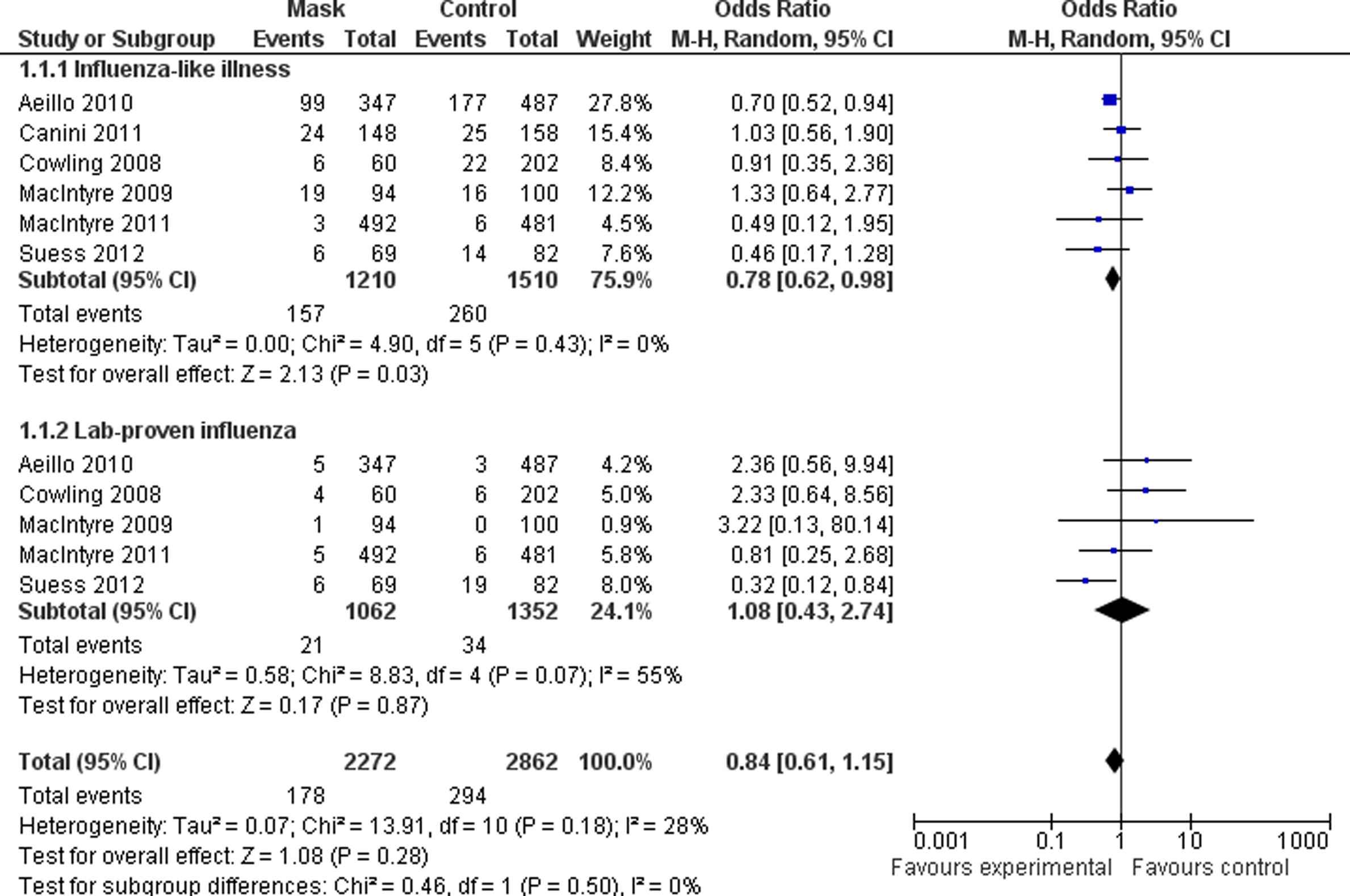
The major limitation of previous studies is their small sample size, and resulting lack of study strength to detect important, albeit moderate (e.g. 40–50%), protective effects of facemasks on laboratory-confirmed influenza or other infections [6,18]. The largest study undertaken to date was a cluster-randomised trial in 509 households, with 2788 recruits; it did not find an additional benefit from the combination of facemasks and hand hygiene over health education (regarding preventive measures) on the overall rate of laboratory-confirmed viral infection, although there was a benefit against secondary transmission of RTI and laboratory-confirmed influenza. The authors pointed to concerns about both under-powering of the study and poor compliance with mask use [11]. Bin Reza et al. recommended that sufficient power may be achieved by larger trials that are multi-centred and run for several years [6]. The authors of this study contend, in addition, that taking important steps to improve compliance with mask use is also critical to inducing a form of herd immunity through widespread mask use. There are considerable logistic and cost issues to consider in the design and conduct of such trials, but the public health benefits of a positive result could be substantial.
Conducting a trial during the Hajj pilgrimage, where approximately 2–3 million people gather annually from all corners of the world and stay for at least 4 days in tents in the Mina valley, offers an excellent opportunity to conduct research in a semi-closed setting.
This study aims to evaluate the efficacy of facemasks in preventing laboratory-confirmed respiratory viral infections and syndromic RTIs. Concurrently, the transmission pattern of influenza at Hajj will be investigated by studying the genetic relatedness between circulating strains using deep genomic sequencing. In addition, the uptake of influenza will be determined and its effectiveness at Hajj will be evaluated using a test-negative case–control design.
2. Methods
2.1. Primary end point
- •
To evaluate the efficacy of facemasks against laboratory-confirmed respiratory viral infections (including influenza, MERS CoV and other respiratory viruses) at Hajj.
2.2. Secondary end points
- •
To investigate the transmission pattern of the influenza virus at Hajj by studying the genetic relatedness between circulating influenza viruses using deep genomic sequencing.
- •
To determine the self-reported influenza vaccine uptake and evaluate its effectiveness at Hajj using a test-negative case–control design.
2.3. Study design
A large-scale cluster-randomised controlled trial has been planned to compare the ‘supervised use of facemasks’ versus ‘standard measures’ (where some may choose to use a facemask) among pilgrims during three consecutive Hajj pilgrimages. Cluster-randomisation to each arm will be in a 1:1 ratio and will be done according to accommodation tents. Computer-generated random numbers will be generated by an offsite research coordinator who will not take part in the recruitment or assessment of participants. The randomisation will be stratified by gender and country of residence to ensure a balanced and proportionate recruitment. The key steps of the study are shown in Fig. 2 .
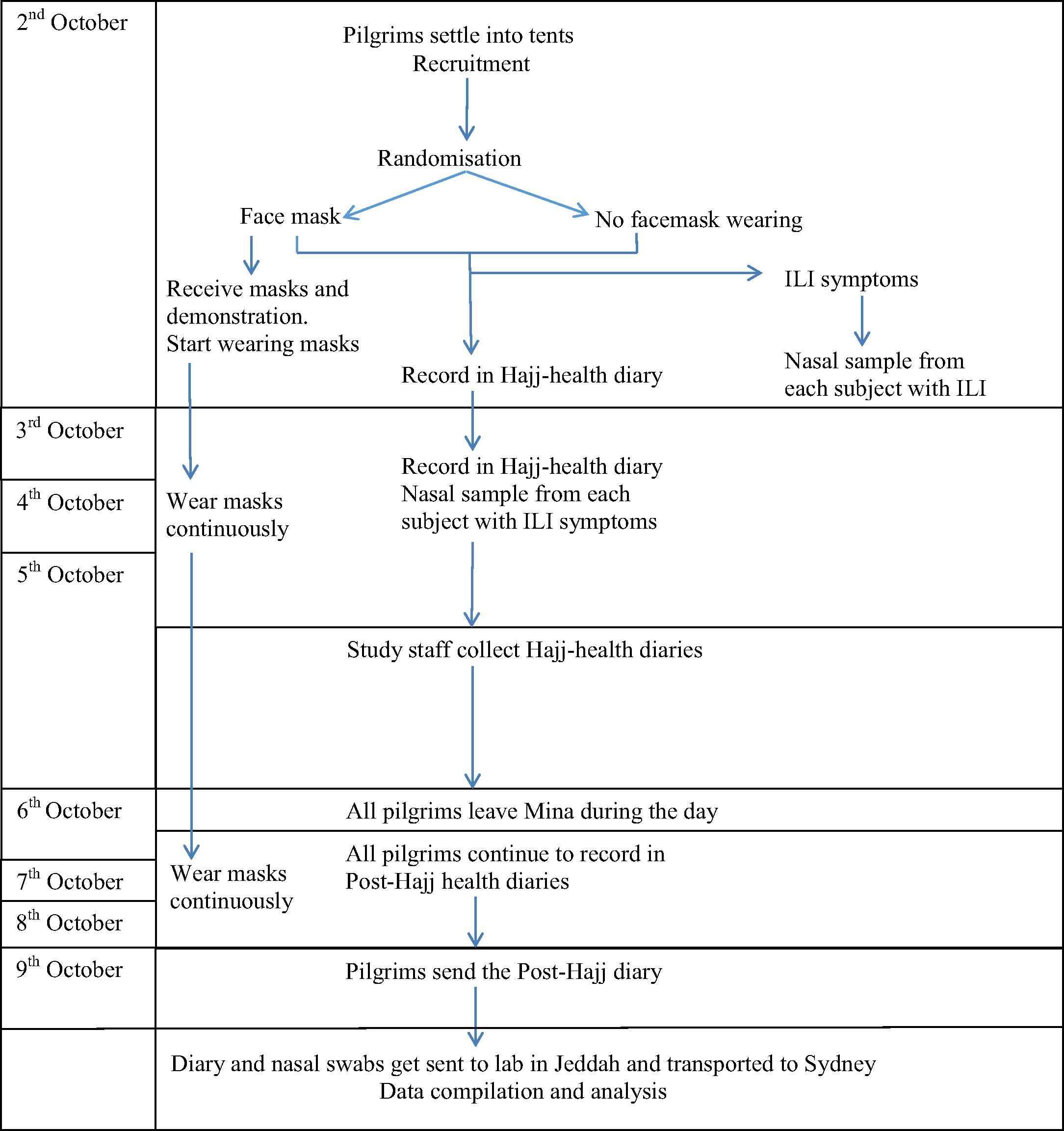
Key steps of the study at the Hajj 2014 (these key steps will remain the same in 2015 except that the recruitment dates will advance by 11 days).
2.4. Recruitment
At the 2013 Hajj, over 1000 pilgrims were recruited from Saudi Arabia, Qatar and Australia, whereas in 2014/2015, participants will also be recruited from at least some of the following countries: Indonesia, Malaysia, Turkey, Morocco, India, Pakistan and Bangladesh (Table 1).
| Countries | Total number of pilgrims | Number of recruits | |
|---|---|---|---|
| 2013 | 2014 | ||
| Saudi Arabia | 1,000,000 | 2,200 | 2200 |
| Indonesia | 200,000 | – | 500 |
| India | 170,000 | – | 450 |
| Pakistan | 150,000 | – | 350 |
| Turkey | 120,000 | – | 300 |
| Bangladesh | 65,000 | – | 200 |
| Morocco | 32,000 | – | 100 |
| Malaysia | 28,000 | – | 100 |
| Australia | 5000 | 200 | 200 |
| Qatar | 2000 | 100 | 100 |
| Total | 1,770,000 | 2500 | 4500 |
Proposed number of pilgrims from each participating country.
Before the commencement of Hajj, all tour groups will be provided with detailed information about the study while in their own country and encouraged to participate. At Hajj, trained study staff will approach tour group leaders in each tent for recruitment on the first day of the pilgrims’ stay in Mina. The research team members will explain the study in detail to the assembled pilgrims and invite them to participate in the study. If they agree, informed consent will be obtained and a baseline questionnaire will be completed which will include data on demographics and clinical information, such as recent respiratory symptoms (Appendix A). Participants and caregivers will not be blinded to the randomisation and will be informed about their allocation (supervised facemask wearing or standard care); however, laboratory staff and statistical analysts will be blinded to the allocation.
2.5. Inclusion and exclusion criteria
2.5.1. Inclusion
- •
Pilgrims from participating countries staying in their respective tents.
- •
Age ⩾ 18 years.
- •
The participant should be able to provide signed informed consent.
2.5.2. Exclusion
- •
Age < 18 years.
- •
Participation in another clinical trial investigating a medical intervention that may interfere with the study outcome measures, like laboratory-confirmed viral RTI.
- •
Any known contraindication to mask use (e.g., allergy to standard [surgical] mask materials).
- •
Refusal/or inability to sign the consent form.
2.6. Data collection
For the tents assigned to mask use, 30 facemasks will be provided to each participant, to be worn continuously (24/7 if possible), and replaced every six hours (sooner if wet), for use during the 4 days at Mina, including Arafat, and preferably for the additional 3 days after leaving Mina.
The standard facemask for this study is ‘3M™ Standard Tie-On Surgical Mask, Cat No: 1816’. Masks may be briefly removed for eating, praying, washing, brushing teeth, or if the pilgrim experiences discomfort. Oral and written information about the instructions for correct mask wearing and disposal will be provided to the mask group (Appendix B). Study staff will practically demonstrate the correct method of how to wear a mask and also help pilgrims put on their masks for the first time. Small plastic bags will be provided for mask disposal and subjects will be instructed to discard the plastic bag containing used masks into local waste bins. A subset of used masks will be saved for further testing by multiplex viral PCR.
A research team member will visit tents each morning and evening to distribute additional facemasks should pilgrims require extra, and to document the development of any reported RTI symptoms.
Study staff will inter alia record each tent number, the number of people recruited, and the total number of people in each tent. Pilgrims will be provided with a Hajj Health diary (Appendix C) for the 4 days at Mina (corresponding to 2014 with 2–5 October) and a second diary, hitherto named post-Hajj Health diary (Appendix D), for the next 3 days after leaving Mina. Each diary will have a unique identifying barcode linked to the study participant number. The diaries (Appendices C and D) will contain questions on demographics, medical conditions, and influenza vaccination history. Each participant, whether in an intervention or a control tent, will provide information about the presence or absence of respiratory symptoms and fever in the diary every day, whenever it is most convenient (morning or evening). Pilgrims will also record in their diaries the amount of time they have worn the mask, the number of masks used that day and whether they were wearing one during their sleep overnight. In addition to English, the study documents (diaries and questionnaires) will be available in various languages, including Arabic, Bahasa Indonesia and Bahasa Malaysia, and the multilingual research team members will remain available to help the participants who do not speak English.
Masks will not be provided to anyone in the control tents, although pilgrims may use their own supply of masks.
The Hajj Health diary (Appendix C) will be collected on the evening of the fourth day. At the same time, the post-Hajj Health diary (Appendix D) will be distributed to these pilgrims with self-addressed prepaid envelopes. Pilgrims will be instructed to post these diaries to the researchers or tour operators may collect them on behalf of the study. Pilgrims whose Hajj Health diaries (Appendix C) were not collected on the fourth day will be instructed to return them together with their post-Hajj Health diaries (Appendix D) in the same envelope.
2.7. Specimen collection and testing
In both groups, the study staff will actively search for pilgrims suffering from RTI (defined as subjective or measured fever and at least one respiratory symptom such as cough, sore throat and rhinorrhoea) twice a day. A nasopharyngeal (NP) swab (or throat swab if an NP swab is too difficult to obtain) will be collected from symptomatic pilgrims by a trained staff member for later molecular diagnostic testing to detect respiratory viruses. The swab used will be a Copan Nylon® Flocked nasal swab in a viral transport medium.
As a part of the routine care, pilgrims in both groups will be supplied with generic medications (such as acetaminophen and ibuprofen) for fever or aches.
The swabs will be stored within 2–3 h of collection at −20 °C and later shipped for molecular diagnostic testing at the Centre for Infectious Diseases and Microbiology Laboratory Services (CIDMLS), Westmead Hospital, NSW, Australia. Pilgrims will be asked to provide their email address, phone number and mail address if they wish to receive the result of the laboratory test. Nucleic acid testing using a multiplex reverse transcriptase polymerase chain reaction (RT-PCR) for influenza A and B, MERS-CoV and other coronaviruses, parainfluenza viruses (types 1, 2, 3 and 4), RSV A and B, adenoviruses, human metapneumovirus (hMPV), and picornaviruses will be performed on nasal swabs.
2.8. Transmission dynamics
To determine the transmission patterns of influenza viruses among Hajj pilgrims, complete genome sequence of influenza viruses will be obtained using a next-generation deep sequencing protocol – using the Illumina MiSeq sequencer available at the Westmead Hospital – which will generate multiple reads per host (∼1000× coverage per host). All sequence data generated will be assembled and aligned using the Geneious (http://www.geneious.com) and VICUNA packages [22], with downstream phylogenetic (and other evolutionary) analysis undertaken using the Geneious, Seminator [23], PhyML [24] and BEAST packages [25]. With these data in hand, it will be possible to investigate: (i) whether the study participants were infected prior to or during the Hajj, (ii) whether there was direct viral transmission among the study participants (such that they harbour very closely related ‘majority’ and ‘minority’ genetic variants), and (iii) if direct transmission is established, whether this occurs more frequently in the mask versus control groups.
Respiratory samples will be stored for 2 years to revalidate any results, and then discarded according to the standard operating procedure of CIDMLS.
2.9. Follow up
All participants will be followed up (e.g. by telephone, mail, email) in their country of residence to collect their post-Hajj Health diaries (as well as Hajj Health diaries if not collected earlier). Consent to study participation allows for the contact of GPs/family physicians (if available) to validate any clinical detail, including vaccination history and subsequent infection, GP consultation, or hospital admission.
2.10. Data analysis
Data available from questionnaires, diaries and laboratory tests will be analysed anonymously to examine whether mask use makes a significant difference in reducing the frequency of laboratory-confirmed respiratory viral infection (including influenza, coronavirus or other respiratory viruses) or syndromic RTI. The primary endpoints (efficacy of facemasks against RTI and laboratory-confirmed viruses respectively) will be analysed by intention-to-treat.
The self-reported uptake rates of influenza vaccination will be determined and vaccine effectiveness will be estimated based on the case-negative case–control methodology.
The results from the genomic sequencing of the influenza virus will assist us in understanding the genetic relatedness of circulating influenza strains at Hajj and the transmission pattern of influenza among pilgrims.
2.11. Sample size calculation
Assuming a moderate intra-cluster correlation of 10% and a mean of 75 participants per cluster (tent), and inflating the sample by a moderately large factor of 8.4 to account for clustering, the sample size required for a cluster-randomised controlled trial to detect a reduction in laboratory-confirmed viral respiratory infection from 12% to 6% with 80% power at 5% significance is 2976 per arm. An additional inflation factor of 1.18 will allow for up to a 15% loss to follow-up, loss due to a priori infection with high penetration or incomplete outcome data. These results in a sample size of approximately 3500 participants per treatment making a total of 7000.
For the first primary outcome (clinical/syndromic RTIs) a smaller sample would be sufficient to answer a more generic question, namely prevention of symptomatic RTI. The sample size required for detecting a reduction from 30% prevalence of RTIs to 15% with 80% power at 5% significance and adjusting for clustering and loss of follow-up is about 1170 participants per treatment making a total of 2340 [26,27].
To achieve the full sample size, the aim will be to conduct the study over three years. It will be attempted to recruit a proportionate (or balanced) number of pilgrims from each participating country according to the number of pilgrims attending Hajj from these countries (see Table 1).
2.12. Ethical implication
The trial has received approval from an Australian Human Research Ethics Committee (NSW HREC Ref: HREC/13/HNE/265), and the Joint Institutional Review Board (J-IRB) of Hamad Medical Corporation - Weill Cornell Medical College in Qatar (IRB Number: 13-00039). The study coordinators of each participating country will provide a modified Ethics Committee submission for collaborators and assist them in applying for ethical approval from the relevant Committees. Only participants who provide written informed consent to take part will be included in this study. Participants will be told that taking part in this study would be voluntary and confidential. All participants will be assigned a study identification (ID) number. Data will be collected and stored under this number only, so that all data are stored anonymously using study ID number. Hard copies of the questionnaires will be stored securely in a locked filing cabinet in locked offices and electronic data will be stored in password-protected computer files. Only members of the research team will have access to the data. The hard copies will be retained for at least 15 years (or more, if the data management protocol of the participating country requires so) and will be disposed according to standard data management guidelines.
3. Discussion
If conducted successfully, this study will be the largest trial assessing the efficacy of standard facemasks against RTIs (including influenza), and thus would add to the evidence compendium on physical interventions for response to an influenza pandemic. In addition, this will be one of the largest studies to be conducted at Hajj involving extensive international collaboration. Additionally, this study will address the newly emerged MERS-CoV threat and the potential role of facemasks in its prevention. It is also hoped to involve international collaborators in studying the burden of nasopharyngeal acquisition of antibiotic-resistant colonising microbes, and assessing the impact of facemask use on the prevention of such infections.
However, there are many challenges. An efficient orchestration of the trial is needed to achieve the study objectives; this include training a large number of study research volunteers (n = 500), leading the team to conduct the trial, appropriate data collection, and storage and transport of samples in optimum temperature. Additionally, the compliance with facemask use in intervention tents must be optimised. Some “contamination” may occur whereby subjects in control tents may use facemasks. These issues would be addressed by analysing data with respect to compliance of facemask use, in addition to intention-to-treat analysis. Study staff will visit the intervention tents twice a day to encourage mask use and advise people if they have problems with wearing facemasks. Study staff will similarly visit the control tents and encourage the recruits to strictly maintain a record of any facemask use, to complete their diary and to collect samples from symptomatic pilgrims. A recent pilot study showed that high compliance could be achieved, up to 76%, where facemask usage was recommended and the purpose explained [28].
The MERS-CoV threat may impact this study in another way. While it highlights the significance of this trial in investigating respiratory diseases at Hajj, the Saudi Arabian Ministry of Health has recommended that those at high risk of MERS infection (e.g. those with chronic medical conditions, pregnant or aged 65+ years) postpone their pilgrimage and that those who perform Hajj use facemasks in crowded places and amongst coughing pilgrims [29]. This means that the confounding behaviour of controls will be carefully measured and included in multiple regression analysis.
All diaries may not be returned despite the sincerest of efforts. Pilgrims are required to post the second set of diaries (post-Hajj Health diaries) in reply-paid self-addressed envelopes, which they might not do on time. To optimise the return of the diaries, their study coordinators in respective countries will send phone/mail reminders to the recruits, and tour operators will assist in diary collection.
Data quality may not be optimum as it relies on self-reported symptoms. Reporting of RTI symptoms may be dictated by the patient’s interests: patients may report more specific symptoms to their physicians to get a prescription for antipyretics/pain killers. Study staff will frequently monitor this, but continuous monitoring will not be feasible.
In addition, the short follow-up duration of this trial means there is a possible failure to detect some infections. The incubation period of respiratory viruses may range from less than 2 days (e.g., rhinovirus and influenza) to more than 5 days (adenovirus, MERS-CoV) [30,31]. However, it is believed that post-Hajj Health diaries will reduce this shortcoming to some extent.
Despite these limitations, if successful, this project will provide valuable data on the efficacy of simple facemasks in preventing viral respiratory infection in Hajj pilgrims. It is believed that this data may be useful to other mass gatherings, such as the Olympics, and perhaps to other closed settings such as hospitals, schools, airplanes, ships and airport settings. It also represents a powerful opportunity to study precise patterns of virus transmission through detailed comparison of circulating viral strains. It is hoped that the outcomes of this study will inform global health authorities about the effective control of respiratory viral infection.
Conflict of interest
Professor Robert Booy and Dr. Leon Heron have received funding from Baxter, CSL, GSK, Merck, Novartis, Pfizer, Roche, Romark and Sanofi Pasteur for the conduct of sponsored research, travel to present at conferences or consultancy work; all funding received are directed to research accounts at The Children’s Hospital at Westmead. Dr. Iman Ridda has received Grants for investigator-driven research from GSK and for consultation from Merck. The other authors have declared no conflict of interest in relation to this work.
Acknowledgments
This project is being funded by a Grant from the Qatar National Research Fund (NPRP 6 – 1505 – 3 – 358). Dr. Iman Ridda is supported by the Australian National Health and Medical Research Council (NHMRC) Training Fellowships (630739), and Professor Edward Holmes is supported by an NHMRC Australia Fellowship.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jegh.2014.08.002.
References
Cite this article
TY - JOUR AU - Mandy Wang AU - Osamah Barasheed AU - Harunor Rashid AU - Robert Booy AU - Haitham El Bashir AU - Elizabeth Haworth AU - Iman Ridda AU - Edward C. Holmes AU - Dominic E. Dwyer AU - Jonathan Nguyen-Van-Tam AU - Ziad A. Memish AU - Leon Heron PY - 2014 DA - 2014/10/02 TI - A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims JO - Journal of Epidemiology and Global Health SP - 181 EP - 189 VL - 5 IS - 2 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2014.08.002 DO - 10.1016/j.jegh.2014.08.002 ID - Wang2014 ER -
