Risk Factors for Severe Pneumonia According to WHO 2005 Criteria Definition Among Children <5 Years of Age in Thai Binh, Vietnam: A Case–Control Study
- DOI
- 10.2991/jegh.k.191009.001How to use a DOI?
- Keywords
- Severe pneumonia; risk factor; children; Thai Binh; Vietnam
- Abstract
Vietnam is one of the 15 countries where the prevalence of child pneumonia is highest. It is a major cause of admission in pediatric hospitals. However, little is known on the burden of severe pneumonia and their risk factors in children <5 years of age in Vietnam. A case–control study was conducted among children aged 2–59 months presenting with pneumonia at the Pediatric Provincial Hospital of Thai Binh. Cases were children with severe pneumonia while controls included those with non-severe pneumonia as defined by the World Health Organization (WHO) classification of 2005. Eighty-three cases and 83 controls were included. Sex ratio was 2.19. Children with severe pneumonia were significantly less likely to receive antibiotics preadmission compared to children with non-severe pneumonia [odds ratio (OR) = 0.16, 95% confidence interval (CI) = 0.06–0.42]. The main risk factors of severe pneumonia were a lack of immunization (OR = 4.77, 95% CI = 1.80–12.65), an exposure to cigarette smoke (OR = 3.87, 95% CI = 1.62–9.23), and having a mother with a low level of education. Children with severe pneumonia were 25 times more likely to present with associated measles with p < 0.0001 and five times more likely to present with diarrhea than children with non-severe pneumonia (p < 0.0001). Improving immunization coverage, educating parents about the risks of passive smoking and the recognition of respiratory distress signs, and facilitating early antibiotic access for infants with acute pulmonary disease should reduce the burden of such illnesses. To implement a national, multicenter study about pneumonia in children, more precise inclusion criteria should be chosen, including radiological and/or biological assessment.
- Copyright
- © 2019 Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
“Acute pneumonia is defined as an acute respiratory infection affecting the lungs” [1]. This is a leading cause of morbidity and mortality, particularly in children younger than 5 years with 704,000 deaths and 60.6 million Disability-Adjusted Life Years (DALYs) based on data from 2015 [2]. The annual incidence of clinical pneumonia in younger children in low-income countries was estimated at 231 episodes per 1000 children in 2015 [3]. In developing countries, the diagnosis of pneumonia mostly relies on clinical examination due to lacking imaging and laboratory capacities. In 2005, World Health Organization (WHO) proposed that acute respiratory infections be divided into three categories including non-pneumonia respiratory tract infection, pneumonia, and severe pneumonia according to clinical criteria [1]. In countries with high incidence of lung infection, 20% of pneumonia are severe and require hospitalizing [4,5]. About 15% of all deaths in children <5 years of age are due to pneumonia in the WHO Western Pacific region, with more than 75% cases in Cambodia, Laos, Vietnam, China, and Philippines [6]. Antibiotics are effective if administered on time in bacterial pneumonia. However, only 70% of pneumonia cases receive an appropriate antibiotic in the developing world [7]. There is not a single or specific and simple mean to prevent this disease. Therefore, the identification of risk factors for pneumonia and notably for severe disease is essential.
Vietnam is one of the 15 countries where the prevalence of child pneumonia is highest. Pneumonia is a leading cause of admission in Vietnamese pediatric hospitals [8] and represented 11.4–11.9% of total DALYs among Vietnamese children <5 years of age in 2014–2017 [9]. Also, pneumonia accounted for 11% of deaths in younger Vietnamese children in 2015, 10 times more than in developed countries [6]. Malnutrition, nonexclusive breastfeeding, insufficient vaccinations, baby’s low weight, indoor pollution, and overcrowding are known risk factors for severe pneumonia among children <5 years of age [10,11]. Little is known on the burden of severe pneumonia and their risk factors in children <5 years of age in Vietnam. Nguyen et al. [12] showed that the risk factors for poor outcome of pneumonia in children were age <1 year, recent admission with an acute respiratory infection, low birth weight, and recent tuberculosis exposure, while breastfeeding, preadmission antibiotic use, and day care attendance were associated with reduced risk. Moreover, Vietnam is characterized by a great diversity of population and epidemiological situations, and it is likely that risk factors for severe pneumonia may vary from one region to another. It is therefore necessary to investigate the specific situation of each region in order to set up interventions appropriate to the local context. To better understand risk factors for severe pneumonia among young children, we conducted a case–control study in the Pediatric Hospital of Thai Binh Province.
2. MATERIALS AND METHODS
2.1. Study Setting
Thai Binh Pediatric Hospital is a secondary referral hospital in central Thai Binh Province, northeast of Vietnam. In 2014, this hospital had 250 beds. Ill children are directly admitted from home (through outpatient or emergency departments) or referred from district hospitals of Thai Binh Province. Children with severe diseases, who do not improve, are referred to the National Hospital of Pediatrics in Hanoi. The Thai Binh Hospital had no microbiological laboratory during the study period in 2014.
2.2. Pneumonia Classification
We used the 2005 WHO Integrated Management of Childhood Illness case definitions for pneumonia among children <5 years of age that was in use at the time the study was conducted [13]. This clinical operational definition for medical practice is based on clinical symptoms such as cough or difficulty breathing and tachypnea. This classification, including criteria for severe and non-severe pneumonia, is detailed in Table 1.
| Signs | Classified as |
|---|---|
| • Any general danger sign (not able to drink or breastfeed, persistent vomiting, convulsions, lethargic, or unconscious) or | Severe pneumonia |
| • Chest indrawing or | |
| • Stridor in calm child | |
| • Fast breathing* | Pneumonia |
| • No signs of pneumonia or severe disease | No pneumonia: cough or cold |
Two up to 12 months: 50 breaths per minute or more; 12 months up to 5 years: 40 breaths per minute or more.
WHO Integrated Management of Childhood Illness case definitions for pneumonia among children aged from 2 to 59 months presenting cough and/or difficult breathing
2.3. Participants and Study Design
A comparative randomly paired case–control study was conducted among children aged 2–59 months presenting with pneumonia at the Pediatric Provincial Hospital of Thai Binh, Vietnam, from April 1, 2014, to June 30, 2014. Parents or caretakers underwent a questionnaire aiming at identifying risk factors for severe pneumonia.
The conceptual framework of Davies et al. [14] was used to construct the data collection questionnaire applied to both groups: cases (severe pneumonia) and controls (non-severe pneumonia).
The Fleiss formula was used to calculate the sample size. Ratio between two groups was 1, and the confidence level was set at 95% (α = 0.05) and the efficient possibility at 80% (β = 0.20). Nonexclusive breastfeeding during the first 6 months was chosen to calculate the sample size because it is known to be the most important risk factor for severe pneumonia with an odds ratio (OR) = 2.7 [15] and p = 0.83 (83% of controls are exposed according to United Nations International Children’s Emergency Fund) [16]. A minimum sample size of 83 cases and 83 controls was indicated.
The cases were children aged 2–59 months with severe pneumonia. The controls were randomly selected children with matched gender, age, and date of hospitalization, and admitted to the same units but with discharge diagnosis of non-severe pneumonia (Figure 1). Children whose parents denied answering questions or expressed a refusal to participate in the research were excluded from the study.
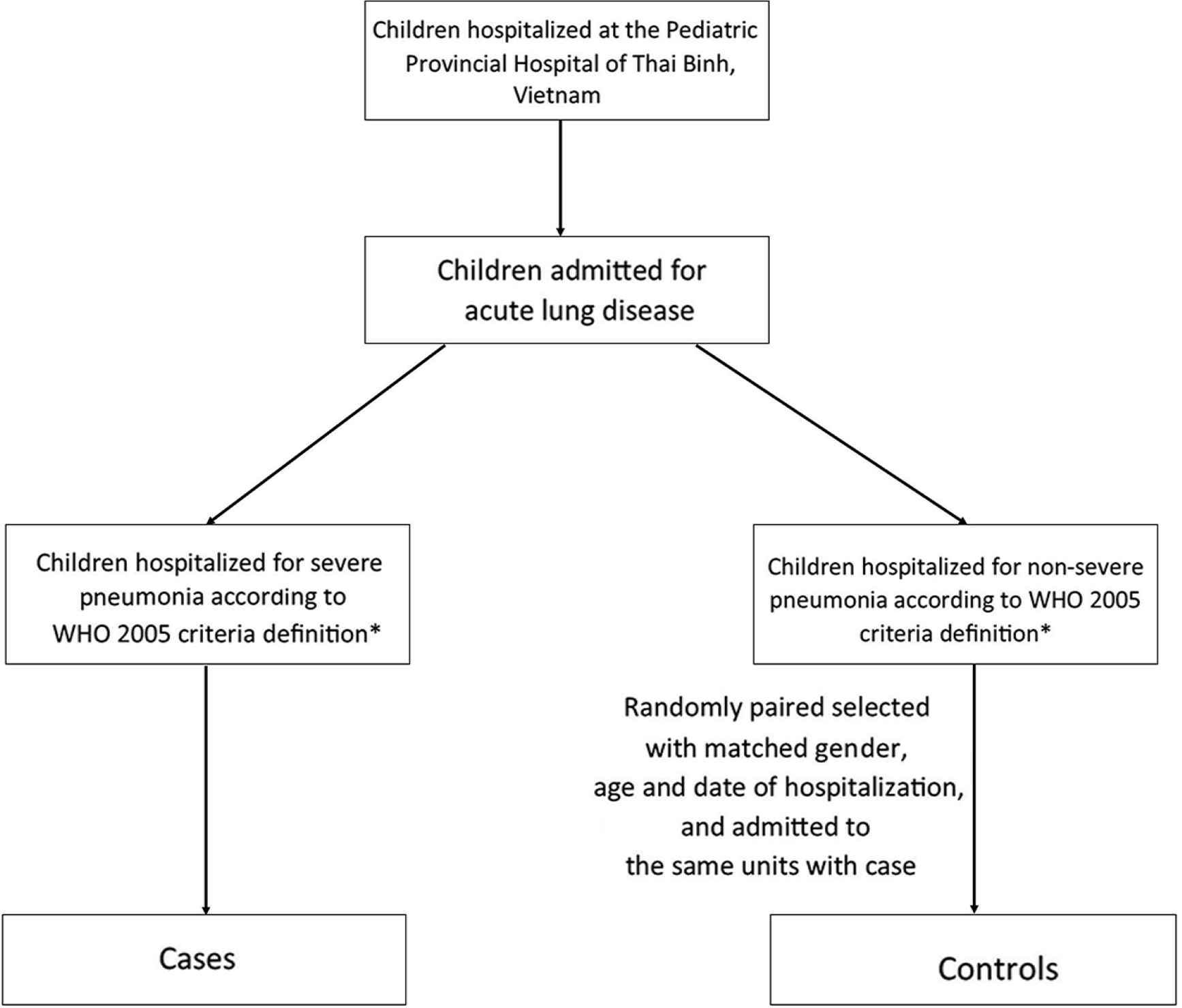
Flow chart of participant recruitment. *Any general danger sign (not able to drink or breastfeed; persistent vomiting; convulsions; lethargic or unconscious) or chest indrawing or stridor in calm child.
All children had one or more digital oximetry measurements in ambient air, a standard chest X-ray, and a complete blood count with C-Reactive Protein (CRP) assay. The chest X-ray was analyzed by a pediatric radiologist. Hypoxia was categorized if peripheral oxygen saturation (SpO2) was <90% [11]. Anemia was retained if the hemoglobin level was <11 g/dL. Time duration between onset of disease and the first contact with the health facilities or with the first use of the antibiotic was expressed in days. The delay in treatment was defined as starting the antibiotics 3 days or more after the first clinical signs or symptoms appeared [17]. According to the expanded program on vaccination in Vietnam [18], tuberculosis vaccine is recommended as soon as possible within 30 days of birth. Three doses of pentavalent vaccine (diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type B) and oral polio vaccine are recommended at 2–4 months. Measles vaccine is recommended at 9–11 months. The lack of immunization was defined by not receiving at least one of the recommended vaccines according to their age. Protein-energetic malnutrition was diagnosed on the criterion of weight/age < −2 SD (standard deviation). Indoor pollution was evaluated on cooking materials (solid wood fuels—coal or nonsolid) and the presence of smokers in the house. The house was considered as a narrow place (overcrowding) if there were more than five people living together. Preterm birth was defined as birth before a gestational age of 37 complete weeks. Low birth weight was defined as a birth weight of <2500 g. Exclusive breastfeeding was defined as the practice of only giving an infant breast milk for the first 6 months of life.
Data were analyzed with Stata ver. 11.1 (Copyright 2009 StataCorp LP; http://www.stata.com). All sociodemographic variables were analyzed to describe the object. Bivariate analysis was designed to compare cases and controls. A Student’s t-test was used to compare means of quantitative variables. Differences in the proportions were tested by Pearson’s chi-square or Fisher’s exact tests when appropriate. The analysis of the relation between risk factors and severe pneumonia was designed to calculate the OR and 95% confidence interval (CI). The Mantel–Haenszel procedure was used to examine the confusion or interaction between factors. The variables with p ≤ 0.1 in the univariable analysis were introduced to the logistic regression of multivariable analysis. This test was used to investigate independently risk factors of severe pneumonia. The test was significative with p-value ≤0.05.
2.4. Ethical Aspects
We obtained the oral informed consent of the participants (parents or caretakers of ill children) after a detailed and complete explanation of the content and purpose of our research. This study was approved by the Ethics Committee of the Hospital for Children of Thai Binh Province (March 20, 2014).
3. RESULTS
3.1. Sociodemographic Characteristics and Clinical Signs
During the period of this study, about 1600 children were hospitalized and 425 (26.6%) were admitted for acute lung disease. Eighty-three cases with severe pneumonia and 83 controls with non-severe pneumonia were included. As expected, no difference was observed between the two groups regarding gender and age. For both cases and controls (n = 166), a male predominance was observed (68.7%) and the sex ratio was 2.19. Pneumonia affected mostly children aged <12 months (80.7 %), with a mean age of 8.1 ± 7.5 months (range 2–51 months) and a median age of 6 months. Forty-three patients (25.9%) resided in Thai Binh city and 123 (74.1%) in narrow villages. No deaths were recorded. Table 2 shows the sociodemographic characteristics of the two groups.
| Characteristics | Cases | Controls | Total |
|---|---|---|---|
| N = 83 (%) | N = 83 (%) | N = 166 (%) | |
| Sex | |||
| Male | 57 (68.7) | 57 (68.7) | 114 (68.7) |
| Female | 26 (31.3) | 26 (31.3) | 52 (31.3) |
| Age group (months) | |||
| <12 | 67 (80.7) | 67 (80.7) | 134 (80.7) |
| 12–24 | 13 (15.7) | 13 (19.3) | 26 (19.3) |
| 24–36 | 1 (1.2) | 1 (1.2) | 2 (1.2) |
| 36–59 | 2 (2.4) | 2 (2.4) | 4 (2.4) |
| Residence | |||
| City | 19 (22.9) | 24 (28.9) | 43 (25.9) |
| Village | 64 (77.1) | 59 (71.1) | 123 (74.1) |
Sociodemographic characteristics of patients
Among 83 children with severe pneumonia, chest indrawing was the most frequent sign (100% of the cases). Twenty-nine (34.9%) children presented with wheezing, 21 (25.3%) had hypoxemia in ambient air, and 19 (22.9%) had central cyanosis. Nineteen patients (22.9%) were unable to breastfeed or drink, six patients (7.2%) had persistent vomiting, and five cases (6.0%) had a consciousness disorder. A total of 16 patients (19.3%) had only chest indrawing as sign of severe pneumonia. Only 27.7% of cases and 34.9% of controls presented fever.
3.2. Blood Testing and Imaging
Seventy (84.3%) severe pneumonia cases and 69 (83.1%) controls had CRP <20 mg/L, and eight cases (9.6%) and eight controls (9.6%) had CRP values ranging from 20 to <50 mg/L. Three cases (3.6%) and five controls (6.0%) had CRP between 50 and 99 mg/L. Only two cases (2.4%) and one control (1.2%) had CRP ≥100 mg/L. We observed no significant difference in CRP values among children with severe pneumonia and non-severe pneumonia. Also, no significant differences in white blood cells count and anemia were observed between the cases and controls (10.074 ± 4.385 mm3 vs 10.338 ± 3.302 mm3, p = 0.21 and 81.9% vs 84.3%, p = 0.34, respectively). Radiological signs of pneumonia (alveolar pneumonia) with point consolidation or presence of infiltrates were found in more than 95% of all the cases. Bilateral bronchopneumonia was the most common sign with no significant difference between severe pneumonia and non-severe pneumonia (80/83, 96.4% vs 79/83, 95.2%, respectively, p = 0.35; data not shown).
3.3. Risk Factor for Severe Pneumonia
Tables 3 and 4 show the results of univariable and multivariable analyses. Children with severe pneumonia were significantly less likely to have received antibiotics before admission compared to children with non-severe pneumonia. Children with severe pneumonia were significantly more likely to lack immunization (five times), to live with smoker(s) (four times), and to have a mother with a level of education equal to (four times) or below that of secondary school (14 times). Finally, children with severe pneumonia were 25 times more likely to present with associated measles and five times more likely to present with diarrhea than children with non-severe pneumonia.
| Variables | Case | Control | OR [95% CI] | p-value |
|---|---|---|---|---|
| N = 83 (%) | N = 83 (%) | |||
| Birth order | ||||
| 1st child in family | 30 (36.1) | 48 (57.8) | Ref | Ref |
| 2nd child in family | 44 (53.0) | 30 (36.1) | 2.34 [1.22–4.50] | 0.01 |
| ≥3rd child in family | 9 (10.8) | 5 (6.0) | 2.88 [0.88–9.42] | 0.08 |
| Education level of mother | ||||
| High school or above | 23 (27.7) | 53 (63.9) | Ref | Ref |
| Secondary school | 40 (48.2) | 27 (32.5) | 3.41 [1.71–6.81] | <0.01 |
| Primary school | 20 (24.1) | 3 (3.6) | 15.36 [4.15–56.85] | <0.0001 |
| Smoker(s) in the family | 53 (63.9) | 24 (28.9) | 4.34 [2.16–8.80] | <0.0001 |
| Indoor air pollutiona | 68 (81.9) | 45 (54.2) | 3.83 [1.80–8.35] | <0.0001 |
| Overcrowdingb | 41 (49.4) | 37 (44.6) | 1.21 [0.63–2.34] | 0.53 |
| Residence = city | 19 (22.9) | 24 (28.9) | 0.73 [0.36–1.47] | 0.19 |
| Acute cough in family | 25 (30.1) | 28 (33.7) | 0.85 [0.42–1.71] | 0.62 |
| Chronic cough in family | 5 (6.0) | 1 (1.2) | 5.26 [0.60–251.54] | 0.09 |
| Lack of breastfeedingc | 39 (47.0) | 30 (36.1) | 1.57 [0.80–3.06] | 0.16 |
| Lack of immunizationd | 36 (43.4) | 14 (16.9) | 3.78 [1.75–8.39] | <0.0001 |
| Low birth weighte | 7 (8.4) | 8 (9.6) | 0.86 [0.25–2.88] | 0.79 |
| Birth history | ||||
| Caesarean | 26 (31.3) | 33 (39.8) | 0.69 [0.36–1.31] | 0.26 |
| Preterm birthf | 11 (13.3) | 10 (12.0) | 1.11 [0.40–3.13] | 0.82 |
| Perinatal asphyxia | 5 (6.0) | 1 (1.2) | 5.26 [0.56–25.54] | 0.09 |
| Congenital malformation | 5 (6.0) | 3 (3.6) | 1.71 [0.32–11.34] | 0.47 |
| Respiratory tract infections during the past 6 months | ||||
| Pneumonia | 29 (34.9) | 14 (16.9) | 2.65 [1.21–5.95] | <0.001 |
| Upper respiratory tract infection | 3 (3.6) | 1 (1.2) | 3.08 [0.24–30.2] | 0.31 |
| Bronchitis | 8 (9.6) | 9 (10.8) | 0.88 [0.28–2.72] | 0.8 |
| Associated infections at the time of admission | ||||
| Measles | 13 (15.7) | 1 (1.2) | 15.23 [2.16–65.77] | <0.0001 |
| Diarrhea | 34 (41.0) | 16 (19.3) | 2.91 [1.37–6.27] | <0.001 |
| Otitis | 6 (7.2) | 2 (2.4) | 3.16 [0.54–32.68] | 0.15 |
| Malnutritiong | 10 (12.0) | 2 (2.4) | 5.55 [1.12–53.24] | 0.02 |
| Antibiotic use before admission | 14 (16.9) | 44 (53.0) | 0.18 [0.08–0.39] | <0.001 |
| Delay >3 days in treatment | 47 (56.6) | 15 (18.1) | 5.92 [2.77–12.89] | <0.0001 |
Indoor pollution was evaluated on cooking materials (solid wood fuels—coal or nonsolid) and the presence of smokers in the house.
More than five people living together.
Lack practice of only giving an infant breast milk for the first 6 months of life.
The lack immunization was defined by not receiving at least one of the recommended vaccines according to their age: tuberculosis vaccine is recommended as soon as possible within 30 days of birth. Three doses of pentavalent vaccine (diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type B) and oral polio vaccine are recommended at 2–4 months. Measles vaccine is recommended at 9–11 months.
Birth weight of <2500 g.
Birth before a gestational age of 37 complete weeks.
Weight/age < −2 SD.
Risk factors of severe pneumonia among children <5 years of age (univariable analysis)
| Variables | Adjusted OR | [95% CI] | p-value |
|---|---|---|---|
| Birth order | |||
| 1st child in family | Ref | ||
| 2nd child in family | 1.94 | [0.77–4.94] | 0.16 |
| ≥3rd child in family | 3.18 | [0.64–15.78] | 0.16 |
| Education level of mother | |||
| High school | Ref | Ref | Ref |
| Secondary school | 4.07 | [1.61–10.24] | <0.0001 |
| Primary school | 13.89 | [3.21–60.06] | <0.0001 |
| Smoker(s) in family | 3.87 | [1.62–9.23] | <0.001 |
| Indoor air pollution* | 2.31 | [0.56–9.58] | 0.25 |
| Chronic cough in family | 12.9 | [0.92–18.11] | 0.06 |
| Lack of immunization | 4.77 | [1.80–12.65] | <0.0001 |
| Perinatal asphyxia | 3.24 | [0.15–14.23] | 0.17 |
| Pneumonia during the past 6 months | 1.78 | [0.23–2.41] | 0.53 |
| Associated diseases | |||
| Measles | 24.89 | [2.41–258.90] | <0.0001 |
| Diarrhea | 4.51 | [1.73–11.77] | <0.0001 |
| Malnutrition | 2.24 | [0.28–17.85] | 0.44 |
| Antibiotic used before admission | 0.16 | [0.06–0.42] | <0.0001 |
| Delay >3 days in treatment | 2.11 | [0.73–6.17] | 0.17 |
Bold, Significant ORs and 95% CIs.
Significant risk factors of severe pneumonia among children aged 2–59 months, multivariable analysis
4. DISCUSSION
In this study, we compare children aged 2–59 months admitted for non-severe pneumonia or for severe pneumonia in a secondary setting in northern Vietnam. Our main results are the following: (1) hospitalization for pneumonia (whatever the severity) primarily affects male patients <1 year of age among children aged 2–59 months; (2) incomplete immunization status, parental smoking, low levels of maternal education, and associated infectious diseases were strongly associated with severe pneumonia as previously documented [10,11]; and (3) use of antibiotics before hospitalization reduces to nearly six times the risk for severe pneumonia.
Vaccination can prevent pneumonia-associated deaths in relation with Streptococcus pneumoniae, Haemophilus influenzae type b infections, measles, influenza, and pertussis [19]. In Vietnam, according to national statistics in 2011, 90% of children were fully immunized for the diseases included in the routine vaccination program with compatible age [16]. In our study, however, the rate was much lower: only 56% of cases and 83% of controls were fully vaccinated. It could account for the frequent association of measles with severe pneumonia in our study. In 2014, in Vietnam, measles accounted for 1.4% of total DALYs (0.25% in 2017) [2], and a total of 130 cases were also reported in our hospital during the first 4 months of 2014 (period of the study), of which about 5% were associated with severe pneumonia (unpublished data). In a study of PrayGod et al. [20], delayed measles vaccination increased by four times the severity of pneumonia among younger children with OR = 3.9, 95% CI = 1.1–14.8, p < 0.0001. Several studies have regarded diarrhea as a significant comorbidity in children suffering severe pneumonia [21–23]. In our patients, the relative risk for diarrhea in severe pneumonia was two times higher than that observed in other studies [24,25]. It is known that many infectious diseases could be associated with loosing stools in children. Conversely, diarrhea contributes to malnutrition and therefore impaired immune response [17], mainly by the occurrence of zinc deficiency [21]. Diarrhea may reduce the digestive absorption of oral antibiotics too and be responsible for treatment failure.
Passive tobacco smoke exposure appeared to be significantly associated with severe pneumonia in our study as observed in other works [26,27]. Passive smoking contributes to pneumonia but also asthma, ear infections, and gross neonatal mortality [28]. Exposure to parental smoking was known as a risk factor for pneumonia-related death among young children [6]. In Vietnam, about 50% of men smoke cigarettes. Household cigarette smoke exposure is very common affecting 70% of preschool children, and 81% of children hospitalized for pneumonia had household cigarette exposure [6,29].
Our study showed that a low level of mother education (primary school) was associated with severe pneumonia in children as previously reported [6,27,30]. Low level of education is one hallmark of poverty, and both are associated with ignorance of diseases and signs of severity, difficulties in the access of health-care facilities, vaccination coverage, delays in treatment and finally poor health status, both in developed and developing world [31]. In Vietnam, for instance, only 5% of mothers are aware about warning signs of severe pneumonia in children [32].
In our patients, delay in antibiotics was significantly associated with severity. For treatment of severe pneumonia, WHO emphasizes on the use of antibiotics and oxygen, including in non-severe pneumonia that requires home therapy with oral amoxicillin [33]. Early antibiotic treatment at home before hospitalization is a very significant protective factor with OR = 0.39, 95% CI = 0.19–0.85, p = 0.02, as shown in a Kenyan study [17]. In a recent study of Nguyen et al. [12] among children hospitalized with pneumonia in central Vietnam, preadmission antibiotic use reduced the risk of severe pneumonia by 1.5 too. In order to allow early antibiotic use if suspected pneumonia, many developing countries, like Pakistan [34], have trained medical officers to antibiotic prescription (oral penicillin A). Community-based management of severe pneumonia cases with oral amoxicillin can reduce the need for referral or unnecessary admission to hospital and the risk of pneumonia complications such as needle-borne infections and unnecessary injections. Oral amoxicillin also reduces the risk of emergence of antimicrobial resistance caused by widespread use of inappropriate second-line antibiotics [34]. However, in our study, children with chest indrawing only were classified as “severe pneumonia.” It is therefore likely that there was a high prevalence of viral infections among children with chest indrawing with/without wheezing and that antibiotics would not have been necessary in these cases. A large case–control study using the new WHO classification to confirm the risk of lack of preadmission antibiotics on severe pneumonia is needed.
Nonexclusive breastfeeding, overcrowding, and low birth weight that are often mentioned as risk factors for severe pneumonia were not significantly associated with severity in our series. It must be said that in Vietnam, since 2013, women are advised to rest during 6 months following delivery, with the expectation of increasing exclusive breastfeeding rates (which was only 17% in 2011) [16]. In addition, birth control for two children per couple leashes time for education and family health. In our series, only 15 children had low birth weight. Various studies have shown that severe malnutrition and a recent history of pneumonia were significantly associated with pneumonia-associated death [35]. These two factors were not significant in our multivariate analysis likely because of a low malnutrition rate (12.5% of severe pneumonia) and the absence of severe malnutrition among children recruited in this study.
Our study has some limitations. The study was conducted in a single provincial pediatric hospital. Our results are therefore only valid in this context and cannot be extrapolated to other places in Vietnam and notably to remote rural areas. It is very likely that, in certain settings, many children with pneumonia are neither recognized nor hospitalized and that deaths related to pneumonia are underreported. Antibiotic use before admission depends on time between symptom onset and hospitalization. Additionally, if children present with respiratory distress, they might admitted to the hospital straight away rather than seeking a private care for antibiotics. We did not take into account the potency of immunosuppression among children, notably HIV infection. In Vietnam, however, HIV screening is only done in at-risk populations because of a low prevalence in the general population (0.4% in 2013 according to WHO [16]). In Thai Binh, the prevalence of HIV infection was (0.1%) in 2014 [36]. Another important limitation was in relation with the definitions of cases and controls. Because our study was conducted from April to June, 2014, we used the 2005 WHO criteria for the management of childhood pneumonia [13]. We therefore included 16 patients with chest indrawing without respiratory distress signs in the severe pneumonia group who were not meeting the current revised 2014 WHO classification criteria. The new classification removed “chest indrawing” as a sign of “severe pneumonia” since it is common in young children with viral infections and not necessarily indicative of severe disease [37]. Moreover, the study did not address microbial etiology. Viral pneumonia (as alveolar bronchiolitis) frequently causes chest indrawing and many bronchiolitis may have been included in both severe and non-severe pneumonia. This is corroborated by the fact that a diagnosis of severe pneumonia correlated with measles and diarrhea, which may indicate a possible viral etiology. For future, radiological findings (alveolar condensation, for instance) and inflammation markers as CRP (as a surrogate of bacterial etiology) should be added to clinical criteria to allow comparison regarding severity among bacterial pneumonia.
5. CONCLUSION
In northern Vietnam, features significantly associated with severity among children aged 2–59 months and hospitalized for pneumonia using the WHO 2005 classification were a lack of or delay in antibiotic use, a lack of immunizations against respiratory infections, a passive exposure to tobacco smokes within the family, a low education level of the mothers, and associated infectious diseases as measles or diarrhea. As a consequence, improving immunization coverage, especially against measles, educating parents about the risks of passive smoking and the recognition of respiratory distress signs, and facilitating early access to antibiotics for infants with acute pulmonary disease should reduce the burden of such illnesses. To implement a broader, national, multicenter study about pneumonia in children, more precise inclusion criteria should be chosen, including radiological and/or biological signs.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
V.T.H., T.L.D., P.M. and P.G. contributed to experimental design, data analysis, statistics, interpretation, and writing. V.T.H. and T.L.D. administered questionnaires and followed patients. D.C.N., N.T.H. and V.N.D. contributed to critically reviewing the manuscript. P.G. coordinated the work.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Van Thuan Hoang AU - Thi Loi Dao AU - Philippe Minodier AU - Duy Cuong Nguyen AU - Nang Trong Hoang AU - Van Nghiem Dang AU - Philippe Gautret PY - 2019 DA - 2019/10/25 TI - Risk Factors for Severe Pneumonia According to WHO 2005 Criteria Definition Among Children <5 Years of Age in Thai Binh, Vietnam: A Case–Control Study JO - Journal of Epidemiology and Global Health SP - 274 EP - 280 VL - 9 IS - 4 SN - 2210-6014 UR - https://doi.org/10.2991/jegh.k.191009.001 DO - 10.2991/jegh.k.191009.001 ID - Hoang2019 ER -
