Improved Access to Diagnostics for Rhodesian Sleeping Sickness around a Conservation Area in Malawi Results in Earlier Detection of Cases and Reduced Mortality
- DOI
- 10.2991/jegh.k.200321.001How to use a DOI?
- Keywords
- Sleeping sickness; human African trypanosomiasis; rhodesian; rhodesiense; diagnosis; diagnostic; Malawi
- Abstract
Trypanosoma brucei rhodesiense Human African Trypanosomiasis (rHAT) is a zoonotic disease transmitted by tsetse flies from wild and domestic animals. It presents as an acute disease and advances rapidly into a neurological form that can only be treated with melarsoprol, which is associated with a high fatality rate. Bringing diagnostic services for rHAT closer to at-risk populations would increase chances of detecting cases in early stages of disease when treatment is safer and more effective. In Malawi, most of the rHAT cases occur around Vwaza Marsh Wildlife Reserve. Until 2013, diagnosis of rHAT in the region was only available at the Rumphi District Hospital that is more than 60 km away from the reserve. In 2013, Malawi’s Ministry of Health initiated a project to enhance the detection of rHAT in five health facilities around Vwaza Marsh by upgrading laboratories and training technicians. We report here a retrospective study that was carried out to evaluate the impact of improving access to diagnostic services on the disease stage at diagnosis and on mortality. Between August 2014 and July 2017, 2014 patients suspected of having the disease were tested by microscopy, including 1267 who were tested in the new facilities. This resulted in the identification of 78 new rHAT cases, of which six died. Compared with previous years, data obtained during this period indicate that access to diagnostic services closer to where people at the greatest risk of infection live promotes identification of cases in earlier stages of infection, and improves treatment outcomes.
- Copyright
- © 2020 The Authors. Published by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Human African Trypanosomiasis (HAT) is a vector-borne, neglected tropical disease that is endemic in sub-Saharan Africa with more than 60 million people living at risk of infection [1]. It is transmitted by the bite of infected tsetse flies of the genus Glossina. Two subspecies of the protozoan parasite Trypanosoma brucei can cause infections in humans, with distinct clinical and epidemiological features [2]. Trypanosoma brucei gambiense infection leads to a chronic disease (Gambian HAT or gHAT) that is found in western and central Africa. It is an anthroponotic disease that has accounted for more than 98% of the HAT cases reported in recent years. On the other hand, infection with T. brucei rhodesiense is responsible for a more acute pathology (Rhodesian HAT or rHAT) that occurs in eastern and southern Africa. It is a zoonotic disease that affects livestock and wildlife, whereas humans are considered as accidental hosts [2]. Since 2015, Malawi has been reporting the largest number of rHAT cases [3].
Human African trypanosomiasis progresses from an early or first stage that is characterized by the proliferation of trypanosomes in the bloodstream and the lymphatic system to a late or second stage in which parasites invade the central nervous system [4,5]. If left untreated, the disease usually leads to coma and death although some cases of self-cure have been reported for the chronic form of the disease [6]. While disease progression is generally more rapid with rHAT than gHAT, a wide range of rHAT pathologies have been reported from different endemic foci. Whereas more rapid disease progression has been reported in Uganda and Kenya, rHAT tends to manifest as a more chronic disease in south-eastern African countries such as Malawi [7,8]. Treatment of first stage rHAT relies on suramin injections, which are associated with occasional but mild drug reactions. Although new drugs are under development [9], patients in second stage disease are currently still treated with multiple intravenous injections of melarsoprol, an arsenic derivative that causes frequent and severe adverse reactions. In particular, an encephalopathic syndrome has been reported to occur in 8.0% of rHAT patients treated with melarsoprol, with a fatality rate as high as 57% [4]. Therefore, early diagnosis of rHAT is key to enable safer and more effective treatment, and thereby help control disease morbidity and mortality.
In Malawi, cases of rHAT have essentially been reported around three conservation areas, namely, Vwaza Marsh Wildlife Reserve, Nkhotakota Wildlife Reserve, and Kasungu National Park, where a rich diversity of wild animals are potential reservoirs of the disease [2,10]. Various wild mammals have been proposed to play a role as T. brucei rhodesiense reservoirs in Zambia, such as bushbucks, waterbucks, duikers, impalas, giraffes, lions, warthogs, zebras, and buffalos [11], but the actual animal species responsible for disease transmission to humans in Malawi have not been identified. After a sharp decline in the 1990s, the number of rHAT cases reported annually from Malawi has been relatively stable for the past two decades, with an average of 35 cases reported between 1998 and 2017 [12]. Owing to the presence of T. brucei rhodesiense reservoirs in conservation areas, elimination of rHAT from Malawi is not considered to be likely in the foreseeable future.
The vast majority of rHAT cases reported in Malawi occur around Vwaza Marsh Wildlife Reserve. In particular, between 2011 and 2014, the number of rHAT cases diagnosed around the reserve represented 90% of all the cases reported in the country. Strikingly, 91% of the cases identified around Vwaza Marsh during this period were diagnosed when they were already in the second stage of the disease, and 21% of them died during treatment (Ministry of Health of Malawi, unpublished data).
Until 2013, Rumphi District Hospital, located approximately 60 km from Vwaza Marsh Wildlife Reserve, was the only health facility that was equipped to perform diagnosis and treatment of rHAT in that region. It was hypothesized that the high mortality among rHAT patients coming from around Vwaza Marsh was essentially due to poor access to diagnosis, resulting in late identification of cases and initiation of treatment. Thus, bringing diagnostic services for rHAT closer to the populations that are at risk of infection was identified as a potential strategy to reduce morbidity and mortality associated with rHAT by enabling earlier identification and treatment of patients. This would also reduce the burden on patients of traveling to Rumphi District Hospital.
In 2013, the Ministry of Health initiated a project to strengthen the capacity of health facilities to diagnose rHAT in the close vicinity of Vwaza Marsh Wildlife Reserve, within the villages where communities live. Four facilities (Thunduwike, Malidade, Katowo, and Mwazisi) were selected on the basis of their location and other factors such as the quality of buildings, existing laboratory infrastructure, and electricity supply. The laboratory of Rumphi District Hospital was also upgraded, considering its essential role in diagnosing and treating rHAT patients in the region (Figure 1). The capacity to diagnose rHAT was strengthened by providing new laboratory equipment and supplies, as well as appropriate training. This equipment included microscopes and centrifuges as well as other laboratory supplies, such as micropipettes and consumables. The laboratories were also refurbished and painted as needed. In each facility, two technicians were trained to perform parasitological diagnosis of rHAT, and a refresher training in clinical diagnosis of rHAT was also provided.
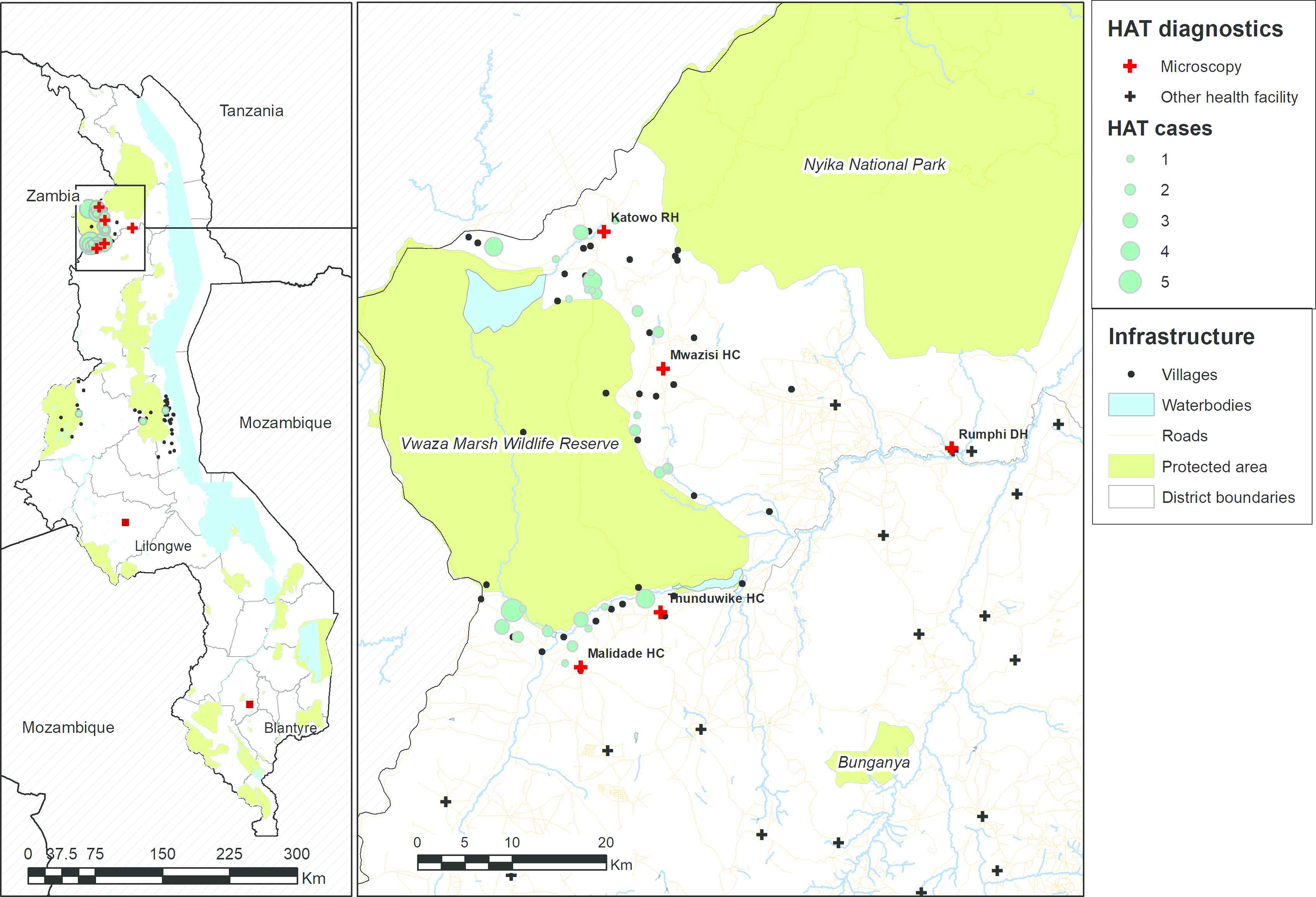
Map showing the villages of residence of rHAT cases that were diagnosed around Vwaza Marsh Wildlife Reserve between 2009 and 2011 (in light blue) and the facilities that were selected for upgrading by the Ministry of Health in 2013 (in red). Other health facilities that were not upgraded are shown in black. Data on protected areas are from the World Database of Protected Areas [22], data on waterbodies and roads are from OpenStreetMap [23], and boundary data are from GADM [24].
We describe here a retrospective study that was performed to evaluate the impact of strengthening the capacity for diagnosis of rHAT in health facilities located close to Vwaza Marsh Wildlife Reserve on the stage at which rHAT cases are diagnosed and on mortality.
2. MATERIALS AND METHODS
2.1. Study Design and Objective
A retrospective analysis of the data collected by the HAT Control Programme of the Ministry of Health of Malawi as part of its routine disease control activities was performed. The analysis included data on screening of clinical suspects, confirmatory diagnosis of rHAT, and treatment outcome between January 2011 and July 2017 around Vwaza Marsh Wildlife Reserve. The objective of the study was to evaluate the impact of the project initiated in 2013 by the Ministry of Health to strengthen capacity for diagnosis of rHAT in this region on the disease stage at diagnosis and on mortality.
2.2. Diagnostic Procedures and Treatment
The following diagnostic procedures were followed in the five upgraded facilities, in accordance with the routine procedures of the Ministry of Health. Any patient presenting with clinical signs and symptoms suggestive of rHAT (including disorders in speech, behavior, walking and sleep, convulsions, headache, backache, fever, and weakness) was tested using the following parasitological methods, which were performed sequentially as long as no positive result was obtained. First, two thick blood smears were prepared; one was stained with Giemsa and the other with acridine orange, as described previously [13–15]. Slides were examined using a Primo Star iLED microscope (Carl Zeiss GmbH, Germany); the slide stained with Giemsa was examined using bright field light, whereas that stained with acridine orange was examined using fluorescence illumination. The capillary tube centrifugation test was then performed using four capillary tubes filled with approximately 75 μl of blood as described previously [16]. Finally, a thin smear was prepared after performing the red blood cell lysis and concentration procedure as described in Biéler et al. [13], stained with acridine orange, and examined under fluorescence illumination.
Any patient found to have trypanosomes using one of the above parasitological methods was referred to Rumphi District Hospital where the stage of the disease was determined and treatment was provided. Staging was performed according to standard procedures, as described by the World Health Organization, by performing a lumbar puncture and examining the Cerebrospinal Fluid (CSF) for the presence of trypanosomes, and counting the number of White Blood Cells (WBCs) [17]. Counting was performed using a Fuchs–Rosenthal chamber and results were expressed as number of WBCs per microliter of CSF. Patients with <6 WBCs/μl and with no trypanosomes in the CSF were considered as first stage cases and were treated with suramin, whereas those with >5 WBCs/μl and/or with trypanosomes in the CSF were considered as second stage patients and were treated with melarsoprol, as per standard procedures [17]. The same criteria for disease stage determination were used throughout the study period.
2.3. Disease Progression Scores
A scale of disease progression was used to give a score to each HAT case. On the basis of clinical information on HAT cases that was available from January 2013 to November 2016, a list including the 24 most frequently reported clinical signs and symptoms was shared with two rHAT clinical experts (Wiza Chilongo, Ministry of Health, Lilongwe, Malawi and Charles Wamboga, Ministry of Health, Kampala, Uganda). The experts were asked to provide a value from 1 to 4 based on their clinical experience, where 1 would correspond to signs and symptoms commonly present at the onset of disease, whereas 4 would correspond to signs and symptoms of very advanced disease, close to death. Using these values, a disease progression score was calculated for each HAT case by adding up the values of the clinical signs and symptoms that were reported. Two different scores were calculated for each HAT case using the two sets of values obtained from the experts (Table 1).
| Clinical sign or symptom | Value, expert 1 | Value, expert 2 |
|---|---|---|
| Abdominal pain | 3 | 1 |
| Altered breathing pattern | 3 | 4 |
| Amenorrhea | 4 | 3 |
| Backache | 3 | 2 |
| Chest pain | 4 | 2 |
| Constipation | 4 | 3 |
| Convulsions | 3 | 4 |
| Cough | 3 | 1 |
| Edema | 4 | 3 |
| Fever | 1 | 1 |
| General pain | 1 | 1 |
| Headache | 1 | 1 |
| Hearing difficulty | 4 | 3 |
| Heart burn | 4 | 3 |
| Itchiness | 1 | 2 |
| Leg pains | 2 | 3 |
| Loss of appetite | 2 | 3 |
| Mental impairment | 3 | 4 |
| Neck pain | 3 | 3 |
| Palpitations | 4 | 1 |
| Sleep disorder | 3 | 3 |
| Speech impairment | 3 | 4 |
| Walking disorder | 4 | 4 |
| Weakness | 2 | 4 |
List of the 24 most frequent clinical signs and symptoms identified among rHAT cases between January 2013 and November 2016, and values corresponding to disease progression attributed to each of them by two clinical experts using a scale from 1 (onset of disease) to 4 (terminal stage)
2.4. Statistical Analysis
Statistical analysis was performed using Stata Statistical Software Release 12.1 (StataCorp LP, 4905 Lakeway Drive College Station, Texas 77845-4512, USA) [18]. Proportions of rHAT cases being diagnosed in the second stage of disease as well as proportions of cases dying during treatment were calculated and expressed as percentages. Exact (Clopper–Pearson) binomial confidence intervals were computed for these proportions using a significance level of 5%. Equality of proportions was tested using Stata’s prtest function. Exact Poisson confidence intervals were calculated for mean WBC counts using a significance level of 5%.
2.5. Ethical Considerations
This study was carried out in conformity with the Declaration of Helsinki and was approved by the Ministry of Health of Malawi through its Human African Trypanosomiasis Control Programme. All HAT patients whose data were used in this study were diagnosed and treated in agreement with the national health policy of Malawi and WHO guidelines. Patient data were anonymized before analysis.
3. RESULTS
A total of 2014 patients were identified as rHAT clinical suspects and tested by microscopy at the five upgraded health facilities (Thunduwike, Malidade, Katowo, Mwazisi, and Rumphi) between August 2014 and July 2017. About 63% of the suspects (1267) were tested at one of the four facilities that were newly equipped to perform parasitological tests (Thunduwike, Malidade, Katowo, and Mwazisi), whereas the remaining 37% (747) were tested at Rumphi District Hospital. Among these suspects, 78 rHAT patients were identified and referred for staging and treatment at Rumphi District Hospital. About 36% of the cases (28) were diagnosed by microscopy at the new facilities, whereas 64% of cases (50) were diagnosed at Rumphi (Table 2). The proportion of cases diagnosed at the new facilities increased over time from 20% in 2014 and 2015 (eight out of 41) to 54% in 2016 and 2017 (20 out of 37). Figure 2 shows that most rHAT cases came from villages located in the neighborhood of Vwaza Marsh Wildlife Reserve. Figure 3 shows the number of rHAT cases that were diagnosed in each facility during this period.
| Facility name | Suspects tested | Cases confirmed | Deaths |
|---|---|---|---|
| Rumphi District Hospital | 747 | 50 | 5 |
| Katowo Rural Hospital | 391 | 15 | 0 |
| Malidade Health Centre | 173 | 5 | 1 |
| Mwazisi Health Centre | 359 | 6 | 0 |
| Thunduwike Health Centre | 344 | 2 | 0 |
| Total | 2014 | 78 | 6 |
Numbers of clinical suspects tested and rHAT cases confirmed at each facility between August 2014 and July 2017. The number of rHAT cases diagnosed in each facility who died during treatment is also indicated
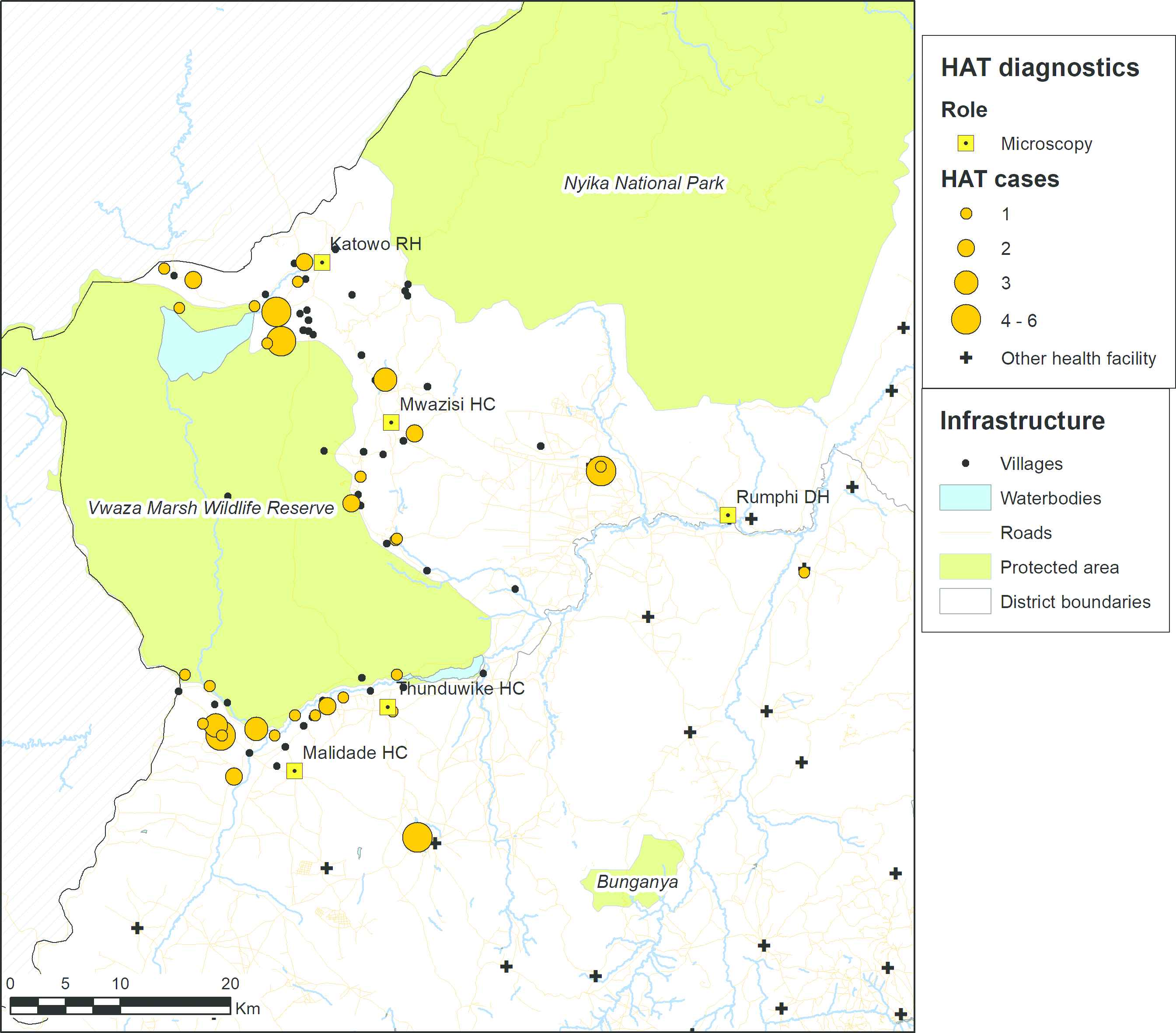
Map showing the villages of residence of the rHAT cases that were diagnosed between August 2014 and July 2017. The size of the orange discs is proportional to the number of cases. Villages from where no cases were identified are represented as black spots. Data on protected areas are from the World Database of Protected Areas [22], data on waterbodies and roads are from OpenStreetMap [23], and boundary data are from GADM [24].
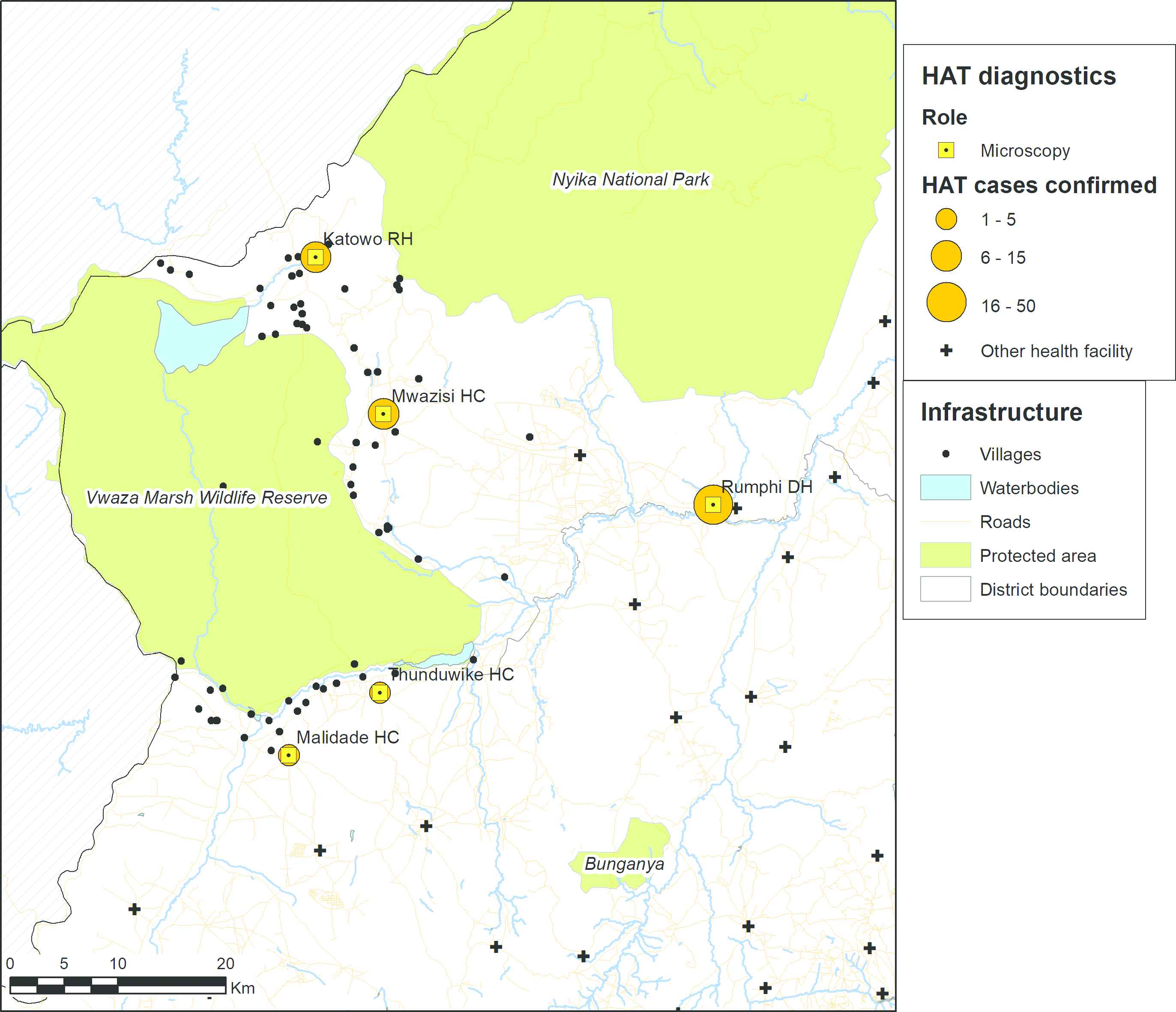
Map showing the health facilities where rHAT cases were diagnosed between August 2014 and July 2017. The size of the orange discs is proportional to the number of cases. Data on protected areas are from the World Database of Protected Areas [22], data on waterbodies and roads are from OpenStreetMap [23], and boundary data are from GADM [24].
A comparison of data obtained during the 3 years that preceded strengthening of facilities (2011–2013) with the 3 years that followed the strengthening (2015–2017) indicated that the availability of upgraded facilities did not result in an increase in the number of rHAT cases that were diagnosed, which remained rather stable (69 cases between 2011 and 2013 and 61 cases between 2015 and 2017; Table 3). In addition, the intervention did not have any impact on the percentage of cases that were diagnosed in second stage, which remained high throughout the study period. Indeed, the annual percentage of cases in second stage fluctuated between 84.2% and 94.1% in 2011–2013 (average of 89.9%) and between 83.3% and 100.0% in 2015–2017 (average of 90.2%). There was, however, a decline in mortality after facilities were strengthened. Whereas the percentage of cases who died between 2011 and 2013 was 20.3% (annual percentage fluctuating between 15.8% and 31.3%), it was only 8.2% between 2015 and 2017 (annual percentage fluctuating between 3.1% and 20.0%). There was some evidence for a significant difference in mortality between these two 3-year periods [12.1%; 95% confidence interval (CI): 0.4–23.8%].
| Year(s) | Cases | Stage 1 | Stage 2 | Stage 2 (%) (95% CI) | Deaths | Deaths (%) (95% CI) |
|---|---|---|---|---|---|---|
| 2011 | 19 | 3 | 16 | 84.2 (60.4–96.6) | 3 | 15.8 (3.4–39.6) |
| 2012 | 16 | 2 | 14 | 87.5 (61.7–98.4) | 5 | 31.3 (11.0–58.7) |
| 2013 | 34 | 2 | 32 | 94.1 (80.3–99.3) | 6 | 17.6 (6.8–34.5) |
| 2011–2013 | 69 | 7 | 62 | 89.9 (80.2–95.8) | 14 | 20.3 (11.6–31.7) |
| 2014 | 32 | 0 | 32 | 100.0 (89.1–100.0) | 7 | 21.9 (9.3–40.0) |
| 2015 | 24 | 4 | 20 | 83.3 (62.6–95.3) | 3 | 12.5 (2.7–32.4) |
| 2016 | 32 | 2 | 30 | 93.8 (79.2–99.2) | 1 | 3.1 (0.1–16.2) |
| 2017 | 5 | 0 | 5 | 100.0 (47.8–100.0) | 1 | 20.0 (0.5–71.6) |
| 2015–2017 | 61 | 6 | 55 | 90.2 (79.8–96.3) | 5 | 8.2 (2.7–18.1) |
Stage 1: rHAT cases diagnosed in the first stage of disease; Stage 2: rHAT cases diagnosed in the second stage of disease. Stage 2 and deaths data are presented both as absolute numbers and as percentages of the numbers of cases diagnosed. Deaths refer to patients dying during treatment at Rumphi District Hospital. Data are presented per year, as well as for the 3-year periods that preceded (2011–2013) and that followed (2015–2017) strengthening of health facilities. CI, confidence interval.
Number of rHAT cases diagnosed, broken down by disease stage, and number of deaths among these cases around Vwaza Marsh Wildlife Reserve between 2011 and 2017
Apparently, there was a lower mortality among rHAT cases referred from the four newly equipped facilities than among the cases diagnosed in Rumphi between August 2014 and July 2017. Out of the 28 cases diagnosed in Thunduwike, Malidade, Katowo, or Mwazisi, only one died (3.6%), whereas five of the 50 cases diagnosed in Rumphi died during treatment (10%) (Table 2). However, this difference was not significant (−6.4%; 95% CI: −17.2%, +4.4%).
Although there was no decrease in the percentage of rHAT cases diagnosed in late stage disease after health facilities were strengthened, we hypothesized that the decrease in mortality could still be explained by earlier diagnosis of rHAT cases, thereby enabling earlier treatment and improved treatment outcome. Available information on clinical signs and symptoms at the time of diagnosis, as well as on WBC counts in the CSF, was analyzed to test this hypothesis. Using information on clinical signs and symptoms, we found that there was a progressive decrease in the average score of disease progression at diagnosis calculated among rHAT cases before facilities were strengthened between 2013 and the first half of 2014. However, no further downward trend was observed after facilities were strengthened in late 2014 (Figure 4).
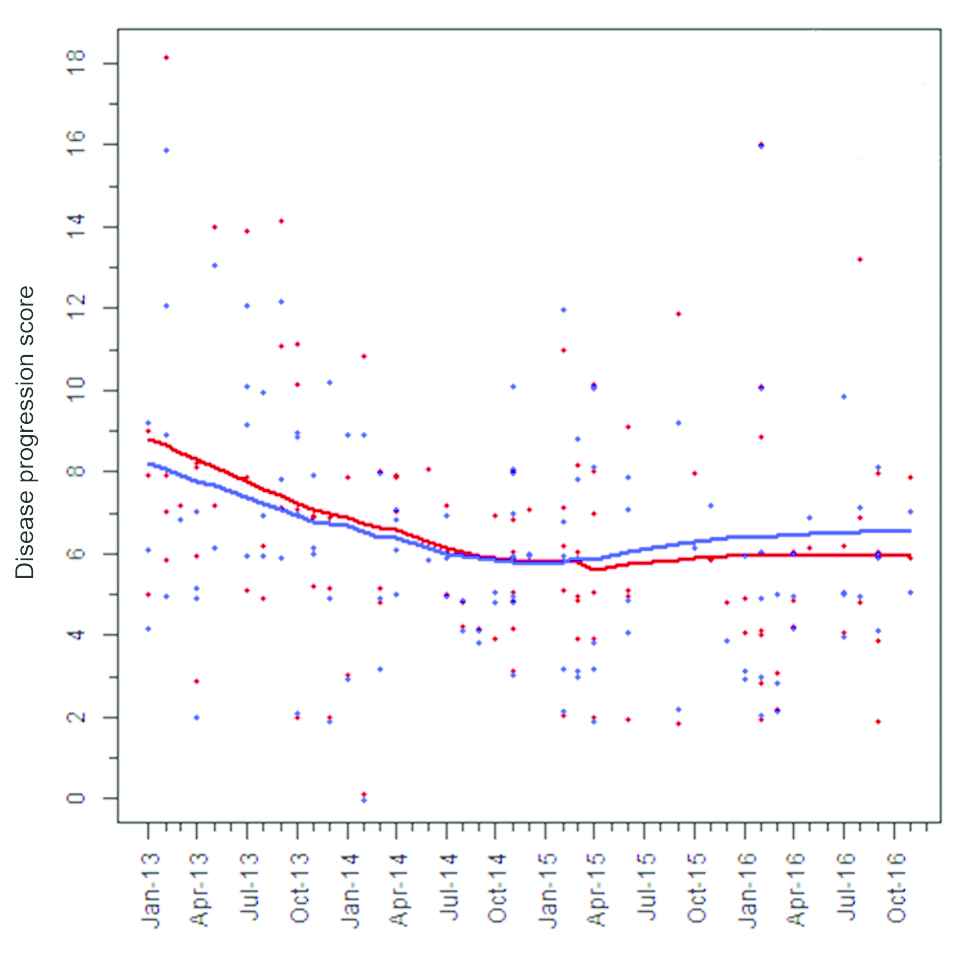
Evolution of disease progression scores among rHAT cases between January 2013 and November 2016 in the study area. The scores of individual HAT cases calculated using values from the clinical experts from Malawi and Uganda are represented by red and blue spots, respectively. The red and blue lines represent the average of these scores.
The evolution of the average number of WBCs counted in the CSF of rHAT cases between 2013 and 2016 was assessed by considering four consecutive periods: two periods of similar length preceding the strengthening of facilities (January 2013–September 2013 and October 2013–July 2014), and two periods of similar length following the strengthening of facilities (August 2014–September 2015 and October 2015–November 2016). Table 4 shows that while the average WBC count was high and increased before facilities were strengthened [81.3 (95% CI: 77.2–85.6) and 99.9 (95% CI: 95.5–104.5)], there was subsequently a progressive and significant decrease [61.4 (95% CI: 58.5–64.3) and 18.5 (95% CI: 16.2–21.0)], when considering all facilities together. The average WBC count was higher in Rumphi [67.0 (95% CI: 63.5–70.6)] than in the other facilities [44.4 (95% CI: 39.6–49.7)] between August 2014 and September 2015, whereas it was lower in Rumphi [7.2 (95% CI: 5.0–10.0)] compared with the other facilities [25.5 (95% CI: 22.1–29.2)] between October 2015 and November 2016. When considering these two periods together (August 2014 through November 2016), there was evidence of a higher average WBC count in Rumphi [55.5 (95% CI: 52.7–58.4)] compared with the other facilities [34.3 (95% CI: 31.4–37.4)]. However, there was a significant number of rHAT cases for which WBCs were not counted, as they had already been identified as second stage patients based on the presence of trypanosomes in their CSF, and this in particular during the last period.
| Period | Facility/Facilities | HAT cases (N) | HAT cases with WBC count data available, N (%) | WBC count mean (95% CI) |
|---|---|---|---|---|
| January 2013–September 2013 | Rumphi | 24 | 18 (75) | 81.3 (77.2–85.6) |
| October 2013–July 2014 | Rumphi | 25 | 19 (76) | 99.9 (95.5–104.5) |
| August 2014–September 2015 | All facilities | 38 | 28 (74) | 61.4 (58.5–64.3) |
| August 2014–September 2015 | Rumphi | 31 | 21 (68) | 67.0 (63.5–70.6) |
| August 2014–September 2015 | Thunduwike, Malidade, Katowo, and Mwazisi | 7 | 7 (100) | 44.4 (39.6–49.7) |
| October 2015–November 2016 | All facilities | 34 | 13 (38) | 18.5 (16.2–21.0) |
| October 2015–November 2016 | Rumphi | 16 | 5 (31) | 7.2 (5.0–10.0) |
| October 2015–November 2016 | Thunduwike, Malidade, Katowo, and Mwazisi | 18 | 8 (44) | 25.5 (22.1–29.2) |
WBC count in the CSF of rHAT cases diagnosed around Vwaza Marsh Wildlife Reserve between January 2013 and November 2016
4. DISCUSSION
The results presented here support the view that strengthening the diagnostic capacity of health facilities in a rHAT endemic region would be associated with a reduction in the distance that patients need to travel to be tested, earlier diagnosis, and better treatment outcomes. Similar conclusions were previously drawn in western Kenya and eastern Uganda, where limited access to appropriate healthcare facilities was identified as a key obstacle to early diagnosis of this disease [19, 20]. While most rHAT suspects presented to the nearer, newly upgraded facilities, a significant percentage of them still preferred to present to the more distant Rumphi District Hospital. This resistance to change in health-seeking behavior was probably due to the fact that Rumphi District Hospital had been known for many years as the only facility capable of diagnosing rHAT in the region. Although an increase in the proportion of cases diagnosed at the new facilities could be observed over time, the results presented here illustrate the challenges to change the behavior of communities when introducing new health services, and highlight the need for sensitization and advocacy efforts directed to populations at risk to achieve high impact.
The clear temporal association between the availability of upgraded facilities for diagnosis and the observed decrease in mortality among rHAT cases would support a causal relationship, although the role of other factors in this decrease cannot be ruled out. While strengthening facilities had no apparent impact on the percentage of rHAT cases being diagnosed in second stage, the finding that there was a progressive decrease in the average concentration of WBCs in the CSF of rHAT cases after facilities were upgraded suggests that cases were being diagnosed earlier in the second stage of disease. The observation that the average WBC count was lower in the upgraded facilities than in Rumphi would also support this view. This could be one of the factors contributing to the decrease in mortality among cases observed. However, WBC counts should be interpreted with caution, as they were not available from all patients and the percentage of cases for which WBC counts were available varied over time, which could have introduced a bias. The fact that mortality was lower among the cases referred from the new facilities than among those diagnosed in Rumphi would also support the view that improved access to diagnostics would result in earlier diagnosis and better treatment outcome. Before facilities were strengthened, it is likely that most of the patients who were eventually diagnosed in Rumphi had first sought treatment in other health facilities that were not equipped to diagnose HAT, resulting in delayed case detection [19,20]. On the other hand, the observation that the average score of disease progression at diagnosis already decreased before facilities were strengthened would suggest that the observed decrease in mortality would be caused by other factors related to earlier interventions. For example, it is possible that the level of awareness of rHAT in communities at risk or among healthcare workers could have increased before facilities were upgraded due to other activities being carried out in the same area. Another explanation could be that patient management at Rumphi District Hospital could have improved through training or changes in personnel, resulting in better treatment outcome.
This study has shown that improving access to rHAT diagnosis can be achieved by strengthening health facilities located close to where patients live, and that this could contribute to earlier case detection, better treatment outcome, and reduced mortality. As access to effective rHAT diagnostic methods is still very limited in most endemic areas located in southeastern African countries, similarly strengthening health facilities in other regions, in particular around conservation areas where T. brucei rhodesiense is known to be endemic, could be considered to help control this disease. However, the observation that there was no increase in the total number of cases diagnosed in the study area as a result of strengthening facilities was somehow surprising and could be interpreted in two different ways. One possibility could be that the health system was already able to capture most rHAT cases before the intervention, albeit with relatively advanced disease. Alternatively, a significantly larger number of health facilities capable of diagnosing rHAT or referring clinical suspects might be required to identify more cases, or population coverage could also be improved using other strategies such as active screening of at-risk populations.
Although there has been significant progress globally towards reaching the target of eliminating rHAT as a public health problem by the year 2020 as defined by the WHO [3,21], the relatively stable number of rHAT cases that has been reported by Malawi for the past 20 years suggests that interruption of transmission might not be within reach in this country. In this context, optimizing disease control strategies in a sustainable manner would be particularly critical, taking into account the cost-effectiveness of interventions as well as their potential for integration in the general healthcare system. Assuming that a significant number of cases are still being missed, more research focusing on the health-seeking behavior of rHAT patients in Malawi could also help inform decisions on the best strategies to be adopted to control the disease. In addition to strengthening diagnosis, other strategies such as intensifying vector control may also need to be explored to progress towards disease elimination. We are hopeful that the positive results that were presented here will help secure the necessary resources to sustain improved access to high-quality diagnosis for rHAT, either from non-governmental or international organizations as part of projects aimed at developing or evaluating new health interventions to control rHAT or from the government of Malawi.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
FUNDING
This work was funded by the
AUTHORS’ CONTRIBUTIONS
JMN and ML contributed in conceptualization. ML and FJ contributed in investigation. SB contributed in project administration. JMN and ML contributed in supervision. FJ contributed in data curation. SB and PB contributed in formal analysis. PB and SB contributed in visualization. SB contributed in the preparation of original manuscript. JMN, ML, FJ, and PB contributed in review and editing of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank the Human African Trypanosomiasis Control Programme of the Ministry of Health of Malawi for sharing the data that were used for this study and acknowledge the personnel of Thunduwike, Malidade, Katowo, Mwazisi, and Rumphi healthcare facilities for their hard work and commitment. We also thank Prof. Enock Matovu (Makerere University, Kampala, Uganda) for training laboratory technicians on microscopy methods, as well as Wiza Chilongo (Ministry of Health, Lilongwe, Malawi) and Charles Wamboga (Ministry of Health, Kampala, Uganda) for sharing their clinical expertise to develop the scale to assess disease progression.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Marshal Lemerani AU - Fredrick Jumah AU - Paul Bessell AU - Sylvain Biéler AU - Joseph Mathu Ndung’u PY - 2020 DA - 2020/03/27 TI - Improved Access to Diagnostics for Rhodesian Sleeping Sickness around a Conservation Area in Malawi Results in Earlier Detection of Cases and Reduced Mortality JO - Journal of Epidemiology and Global Health SP - 280 EP - 287 VL - 10 IS - 4 SN - 2210-6014 UR - https://doi.org/10.2991/jegh.k.200321.001 DO - 10.2991/jegh.k.200321.001 ID - Lemerani2020 ER -
