Real-world Accuracy of Transthoracic Echocardiography in Diagnosing Bicuspid Aortic Valve Morphology: A Single Center UK Experience
- DOI
- 10.2991/artres.k.191219.001How to use a DOI?
- Keywords
- Aortic valve morphology; transthoracic echocardiography; bicuspid aortic valve
- Abstract
Objectives: The aim of this study was to compare the diagnostic accuracy of Transthoracic Echocardiography (TTE) in assessing Bicuspid Aortic Valve (BAV) morphology.
Methods: Case notes of 408 patients (>18 years old) undergoing elective Aortic Valve (AV) surgery over a 2-year period were retrospectively reviewed. Aortic valve data was collected from preoperative TTE reports and intraoperative records. The diagnostic accuracy of TTE for identifying AV morphology was assessed using intraoperative findings as the gold standard. One-hundred-and-eighty-nine (46.3%) patients had a recent TTE with complete morphological assessment of the AV and an operation note documenting AV morphology. Cases that were ‘unable to be determined’ on TTE were included as false negatives.
Results: TTE correctly identified AV morphology in 165 of the patients, equating to an accuracy of 79.1% (sensitivity = 72.4%, specificity = 87.3%). For BAV patients alone, accuracy was 81.3% (sensitivity = 65.8%, specificity = 90.0%), and for Tricuspid Aortic Valve (TAV) patients, accuracy was 77.1% (sensitivity = 75.5%, specificity = 81.4%). There was no significant difference in diagnostic accuracy of TTE for BAV and TAV (p = 0.464), nor was there any difference between valve calcification (p = 0.196) and functional disease (p = 0.088).
Conclusion: Our data suggests that identification of BAV from TTE requires expertise and therefore it is operator dependent. A larger study is required to confirm our findings.
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Bicuspid Aortic Valve (BAV) disease is the most common congenital cardiac abnormality affecting between 0.5% and 2% of people worldwide, and has a male predominance of between 2:1 and 4:1 [1–3]. It occurs when two of the normal three aortic valve (Tricuspid Aortic Valve; TAV) leaflets are fused, or where one leaflet fails to form completely. BAV disease appears to have a genetic basis with familial clustering seen in 10–35% of individuals [4,5]. One of the few genetic mutations identified in BAV families is in the cell signaling gene NOTCH1, a critical pathway in coordination of early valvulogenesis [6,7]. A ‘true’ BAV (Sievers type 0) is one comprising two cusps of equal size orientated in an antero-posterior or lateral position, but is relatively rare (approximately 6% of all BAVs) [8]. More commonly a ‘raphe’ or fusion line is seen between two of the three cusps resulting in a BAV with uneven leaflets, most commonly between the right and left coronary cusps (approximately 70%; Sievers type 1).
Bicuspid aortic valve patients are at increased risk of both aortic valve and vessel disease. Degeneration and calcification of the aortic valve are more frequent, start earlier and progress more rapidly [9–11]. Aortic stenosis not uncommonly presents in the third or fourth decade of life, and regurgitation may occur in up to 40% of BAV patients [12,13]. BAV disease is also associated with an increased incidence of ascending aortic aneurysms (between 30% and 80%) and aortic dissection (eightfold increased risk) [14–16]. Historically, a common genetic defect underlying valve malformation and aortic wall weakness has been suggested; however with recent development of 4D flow Magnetic Resonance Imaging (MRI), evidence is emerging to suggest that altered haemodynamics in the ascending aorta may increase wall shear stress contributing to aortopathy [17–19].
With these complications in mind, it has become increasingly important to identify BAV patients at the earliest opportunity. Failure to do so may lead to mismanagement, including inappropriate surveillance, neglect of progressive valvular and vascular pathologies and increased risk of catastrophic complications. Transthoracic echocardiography (TTE) is recommended for the initial assessment of suspected valvular heart disease according to the 2017 American Heart Association guidelines [20]. Serial imaging of BAV patients is indicated once the ascending aortic diameter exceeds 4.0 cm. Echocardiography should be offered to all first-degree relatives because of an increased incidence of BAV in these individuals [21].
The challenges of correct interpretation of Aortic Valve (AV) morphology on TTE and the implications for potential mismanagement has been published previously, and strategies to assist with correct diagnosis have been described [22,23]. Correct identification of aortic valve morphology is particularly important because criteria for treatment of BAV and TAV patients differ, particularly in regard to aortic replacement for aortic aneurysm [24]. Replacement of the ascending aorta may be considered at 4.5 cm for a BAV patient, but not below 5.5 cm for TAV patients [20]. More recent evidence also suggests that BAV cusp morphology may predict risk of future valve disease and aortopathy [25–27]. Thus, it is vital that aortic valve morphology is accurately assessed and the correct diagnosis of BAV made.
Transthoracic echocardiography is a safe, non-invasive procedure which is sensitive and specific for detecting BAV disease [24]. However, a number of studies have suggested its accuracy may be inferior to other imaging modalities, for example Trans-esophageal Echocardiography (TOE), Computerized Tomography (CT) and MRI [28–30]. Furthermore, heavy calcification which commonly affects BAVs may further reduce the accuracy of TTE [31]. Overall accuracy of TTE for diagnosis of BAV is quoted between 66% and 96% depending on exclusion criteria and extent of valve disease [29,31,32]. However, when quoting accuracy of TTE previous papers have frequently based accuracy on expert review by senior sonographers and consultant cardiologists [28,32,33]. This may have tended to overestimate accuracy since in the majority of center’s images will be reported solo by the duty sonographer. The aim of this study was to generate a ‘real-world’ impression of the diagnostic accuracy of TTE in BAV disease by comparing morphological assessment on non-reviewed TTE reports with intraoperative findings.
2. MATERIALS AND METHODS
Local approval from the trust was obtained to waive the requirement for informed consent as no patient identifiable data has been involved in this study.
2.1. Patient Selection and Data Collection
Local approval was gained to perform a retrospective review of all patients undergoing elective aortic valve replacement with or without an additional procedure in a single surgical referral center in the south of England, between January 2014 and February 2016. Exclusion criteria included transcatheter AV replacement, redo surgery and patients whose TTE was not performed in the study center. Demographic data, the most recent pre-operative TTE report (including the reporter’s profession), the operation note, and any additional imaging were reviewed. Valve morphology and valve disease were recorded from the TTE report and compared with the valve morphology recorded on the operation note, which acted as the gold standard this study.
2.2. Transthoracic Echocardiography
Two-dimensional TTE was performed using contemporary clinical ultrasound systems (ie33 or Epiq 7 ultrasound systems -Philips; Eindhoven, The Netherlands) equipped with S5-1 or X5-1 matrix array transducers. Images were acquired and reported by a range of sonographers and cardiologists of differing experience. TTE studies were performed in accordance with national and international guidelines; in summary, the aortic valve was visualized in parasternal, apical and subcostal views with cusp morphology predominantly assessed in the parasternal long and short axis views. Spectral Doppler imaging was used to document peak and mean pressure gradients across the valve and color Doppler imaging was used to identify aortic regurgitation. The operating surgeons were aware of the morphological diagnosis from the TTE reports as well as the patients’ past medical history and current symptoms.
2.3. Statistical Analysis
Independent sample t-tests were used to assess the differences between patient’s demographics (numerical variables) and chi-squared tests were used to assess the differences between the groups (categorical variables). p < 0.05 was taken as significant. Means are quoted ± standard deviation.
3. RESULTS
3.1. Patient Characteristics
Four hundred and eight patients were included in the study (n = 242; 59.3% male) and mean age was 71.3 ± 11.9 years. The most common operation performed was isolated aortic valve replacement and concomitant coronary artery bypass grafting was the most common secondary procedure (Table 1). The majority of TTE reporting was performed by sonographers (392; 96.1%) and the mean interval between TTE and operation was 154 (±275) days. Isolated severe aortic stenosis was the most frequent functional abnormality at presentation in both TAV and BAV groups (39.8% and 50.0% respectively).
| Demographic | Aortic valve morphology (operative finding) | p-value (TAV vs. BAV) | |
|---|---|---|---|
| Tricuspid | Bicuspid | ||
| Number of patients (n) | 130 | 59 | |
| Age (years) | 71.1 ± 12.5 | 66.5 ± 13.7 | 0.027 |
| Gender (n) | |||
| Male | 72 | 35 | 0.303 |
| Female | 58 | 24 | |
| Procedure (n) | |||
| Isolated AVR | 56 | 33 | 0.457 |
| AVR + CABG | 45 | 11 | |
| AVR + aortic procedure | 9 | 6 | |
| AVR + mitral procedure | 6 | 2 | |
| AVR + other procedure | 1 | 1 | |
| Isolated aortic procedure | 8 | 4 | |
| Aortic procedure + other procedure | 1 | 0 | |
| Other procedure | 4 | 1 | |
| Reporter (n) | |||
| Doctor | 10 | 4 | 0.824 |
| Sonographer | 120 | 55 | |
| Time from TTE to Op (days) | 200 ± 366 | 170 ± 157 | 0.554 |
| Aortic valve disease on TTE (n) | |||
| Isolated stenosis | 52 | 29 | 0.393 |
| Mild | 1 | 1 | |
| Mod | 9 | 3 | |
| Severe | 42 | 25 | |
| Isolated regurgitation | 29 | 10 | |
| Mild | 3 | 2 | |
| Mod | 16 | 4 | |
| Severe | 10 | 4 | |
| Mixed | 48 | 19 | |
| Normal or unknown | 2 | 1 | |
AVR, aortic valve replacement; BAV, bicuspid aortic valve; CABG, coronary artery bypass grafting; TAV, tricuspid aortic valve.
Demographic data for the study population
3.2. TTE and Operative Documentation
Of the 408 patients included, 189 (46.3%) patients had a recent TTE with complete morphological assessment of the aortic valve and an operation note documenting aortic valve morphology. Of the 219 patients without comprehensive documentation, 160 (73.1%) had incomplete morphological assessment documented on TTE, 102 (46.6%) had no documentation at operation and 43 (19.6%) had no documentation of either. Of the 160 patients with operative documentation but inadequate documentation on TTE, 57 (35.6%) had suboptimal views meaning they were ‘unable to determine’ aortic valve morphology. Of these 25 were TAV, 15 were BAV and 18 were not documented intraoperatively (Figure 1).
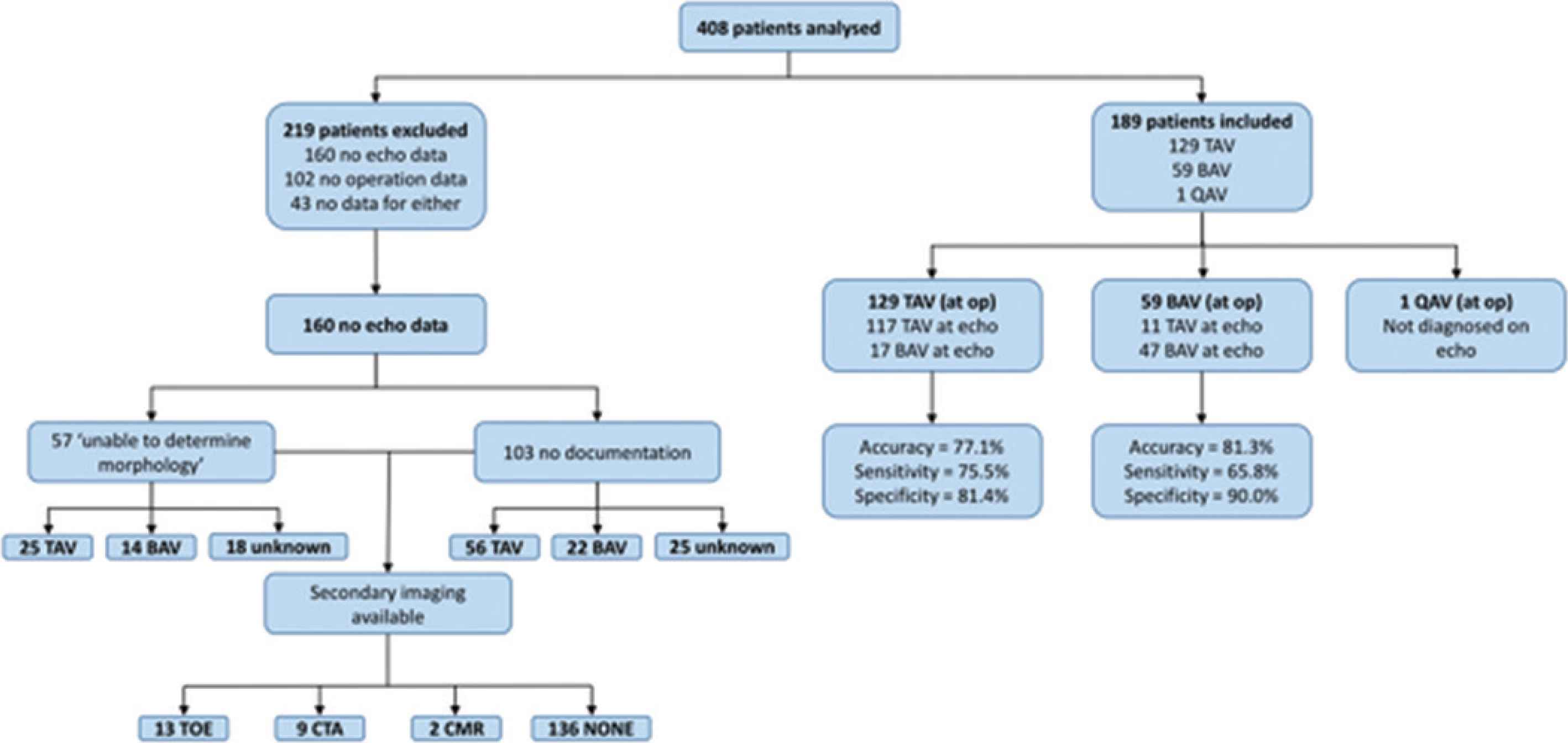
Flow diagram demonstrating reasons for patient exclusion and summarizing paper findings. BAV, bicuspid aortic valve; CMR, cardiac magnetic resonance imaging; CTA, computerised tomographic angiography; QAV, quadricuspid aortic valve; TAV, tricuspid aortic valve; TOE, transoesophageal echocardiography.
3.3. Effect of Valve Morphology on Diagnostic Accuracy of TTE
Transthoracic echo and intraoperative findings were compared for the 189 patients with complete data. Operative findings were used as the gold-standard, and cases that were ‘unable to be determined’ on TTE were included as false negatives. TTE correctly identified AV morphology in 165 of the patients, equating to an overall ‘real-world’ accuracy of 79.1% (sensitivity = 72.4%, specificity = 87.3%). Overall accuracy of TTE for diagnosing BAV in our study was 81.3% (sensitivity = 65.8%, specificity = 90.0%). For TAV, accuracy was 77.1% (sensitivity = 75.5%, specificity = 81.4%; Table 2). There was no significant difference between the diagnostic accuracy of TTE for BAV and TAV (p = 0.464).
| Tricuspid | Bicuspid | |
|---|---|---|
| Accuracy (%) | 77.1 | 81.3 |
| Sensitivity (%) | 75.5 | 65.8 |
| Specificity (%) | 81.4 | 90.0 |
| PPV (%) | 91.4 | 78.7 |
| NPV (%) | 55.8 | 82.4 |
NPV, negative predictive value; PPV, positive predictive value.
Overall accuracy of diagnosing AV morphology
3.4. Effect of Operator Characteristics on Diagnostic Accuracy of TTE
In accordance with this study’s aim to obtain a ‘real-world’ estimate of the accuracy of TTE in diagnosing BAV, imaging was performed by 40 operators of differing experience, and included cardiologists and sonographers. Incidence of ‘indeterminate’ AV morphology were comparable between sonographers and cardiologists (14% and 12.5% respectively). Accuracy for diagnosis of TAV and BAV morphology in the cardiologist group was 86.7% for both, with a sensitivity and specificity of 90.9% and 75.0% for TAV and 60.0% and 100.0% for BAV respectively. Accuracy for diagnosis of TAV and BAV morphology in the sonographer group was 76.4% and 81.2% respectively, with a sensitivity and specificity of 74.3% and 81.8% for TAV and 65.8% and 90.0% for BAV respectively (Tables 3 and 4).
| Cardiologists | ||
|---|---|---|
| Tricuspid | Bicuspid | |
| Accuracy (%) | 86.7 | 86.7 |
| Sensitivity (%) | 90.9 | 60.0 |
| Specificity (%) | 75.0 | 100.0 |
| PPV (%) | 90.9 | 100.0 |
| NPV (%) | 75.0 | 83.3 |
NPV, negative predictive value; PPV, positive predictive value.
Accuracy of diagnosis of AV morphology by cardiologist reporting
| Sonographers | ||
|---|---|---|
| Tricuspid | Bicuspid | |
| Accuracy (%) | 76.4 | 81.2 |
| Sensitivity (%) | 74.3 | 65.8 |
| Specificity (%) | 81.8 | 90.0 |
| PPV (%) | 91.5 | 78.7 |
| NPV (%) | 54.9 | 82.4 |
NPV, negative predictive value; PPV, positive predictive value.
Accuracy of diagnosis of AV morphology by sonographer reporting
3.5. Effect of Valve Disease on Diagnostic Accuracy of TTE
The nature and severity of functional aortic valve disease diagnosed on TTE was categorized (stenosis or regurgitation, severe or non-severe and mixed disease). In addition, data relating to intraoperative assessment of aortic valve calcification was collected. Patients with mixed functional disease demonstrated the highest percentage of correct diagnosis (90.2%), with severe regurgitation demonstrating the lowest correct diagnosis (71.4%). This difference did not reach statistical significance (p = 0.088) Incidence of aortic valve calcification appeared to be higher in patients with incorrect TTE diagnosis compared with correct diagnosis, however this was not statistically significant (92.3% and 76.3% respectively; p = 0.196).
3.6. Identification of BAV Leaflet Configuration
At operation, 28/96 (29.2%) of the BAV patients with operative documentation of AV morphology had leaflet configuration assessed according to Sievers classification. The majority were found to be type 1, with left/right cusp fusion. At TTE, 10/75 (13.3%) of the patients diagnosed as BAV were classified according to Sievers of which two were correctly matched with operative findings (both type 1 with right/left fusion; Table 5).
| Sievers-morphology | n at operation | n at TTE |
|---|---|---|
| Type 0 (true) | 2 | 1 |
| Type 1 (right/left) | 24 | 9 |
| Type 1 (right/non) | 2 | 0 |
| Not classified | 68 | 65 |
| Total | 96 | 75 |
TTE, transthoracic echocardiography.
Numbers of patients classified by Sievers at operation and on TTE
3.7. Second Line Investigation of AV Morphology
Second line investigation with either TOE, computerized tomography angiography or MRI was performed for 24 (15.0%) of the patients whose AV morphology was either not documented or indeterminate at initial TTE (Figure 1).
4. DISCUSSION
The aim of this study was to investigate the diagnostic accuracy of TTE for assessing aortic valve morphology in ‘real-world’ practice. The key findings of this study were: (1) overall ‘real-world’ accuracy for correct identification of BAV and TAV morphology were 81.3% (sensitivity = 65.8%, specificity = 90.0%), and 77.1% (sensitivity = 75.5%, specificity = 81.4%) respectively; (2) there was no appreciable difference in the accuracy of sonographers and cardiologists; (3) there was a trend towards reduced accuracy of TTE when severe regurgitation was present; (4) valve calcification did not make an appreciable difference to diagnostic accuracy; (5) incomplete TTE and operative data was a frequent finding.
The majority of previous studies assessing the accuracy of TTE for diagnosing BAV disease have utilized expert reviewers when assessing images. We hypothesized that this may have tended to overestimate the accuracy of TTE. Therefore, we sought to assess the accuracy of TTE using reports generated at the point of care. Images were acquired and reported by a range of professional including cardiologists and sonographers of varied experience. To the best of our knowledge, ours is the first study to obtain a ‘real-world’ estimate of TTE accuracy, which might be expected in routine hospital practice.
In comparison to our ‘real-world’ estimate of TTE accuracy for BAV disease of 81.3%, previous reports quote lower accuracies of between 56% and 77%. Ayad et al. retrospectively reviewed transthoracic echocardiograms of 100 patients with aortic stenosis. They used two experts to review images and calculated a sensitivity and specificity of 69% and 67% respectively for BAV, and similar figures for TAV (67% and 69% respectively). Alegret et al. [34] demonstrated an accuracy of 89% in TAV and 56% in BAV for TTE with TOE as the standard. In this study, two independent reviewers were used to make image assessments. Yousry et al. [28] compared TTE to TOE with expert reviewers and using operative findings as the standard. TTE sensitivity and specificity for BAV diagnosis were 77% and 82% respectively (TOE was superior at 92% and 94% respectively). Similarly, Takeda et al. [31] quote an accuracy, sensitivity and specificity for the diagnosis of BAV of 77%, 61% and 81% respectively in TTE. However, they did not exclude patients with indeterminate echo findings as was done in the present study. They also found that aortic valve calcification reduced the accuracy of TTE. Furthermore, Malaisrie et al. [29] reported similar accuracy figures to the present study. Of 218 patients, 123 (56%) had a BAV, and of these 76 (62%) were identified preoperatively as BAV, 12 (10%) were misidentified as TAV, and 35 (28%) were non-diagnostic. Therefore our ‘real-world’ accuracy estimate is superior to previous papers which have used expert reviewers.
Incomplete echocardiographic data is a well-documented problem particular in assessment of the ascending aorta [35]. In the present study, we encountered a similar problem with over 50% of TTE reports containing incomplete information relating to aortic valve morphology. Furthermore, approximately 25% of the operation notes did not describe aortic valve morphology. A further 43 patients (10.4%) had no documentation of AV morphology on either pre-op TTE or the operation note. Of these, 23 (6%) had no secondary imaging, and as far as we could ascertain, no record of AV morphology. This may however be an overestimation because other imaging results may have been available through the patient’s general practitioner or peripheral hospitals, which were not acquired in this study. Furthermore, expert review of the TTE images that were ‘unable to determine’ or not documented may have been able to elucidate the AV morphology, increasing the accuracy of TTE further. A limitation of this study was the lack of blinding of the operating surgeons to the TTE reports. Therefore, we cannot exclude the possibility of bias influencing the intraoperative interpretation of AV morphology. In addition, whilst the gold standard for AV morphology assessment in this study was intraoperative assessment, formal histological assessment of valve samples in the laboratory would be an alternative method.
Accurate quantification of BAV morphology is likely to become increasingly important, particularly with emerging evidence suggesting that specific shear stress patterns are associated with differing cusp orientations [36]. BAV configurations may have future implications for the risk of ascending aortic aneurysm. Following this work, education to echocardiography and surgical staff has been provided, and an intervention to include a drop-down menu for documenting AV morphology on the electronic operation note is being investigated to improve practice. We recommend considering alternative imaging methods when TTE is inconclusive.
5. CONCLUSION
‘Real-world’ accuracy of TTE for diagnosis of BAV disease reported in the present study appears to be superior to previous estimates which have been based on expert review. Where TTE diagnosis is not possible, we strongly recommend a second imaging modality to ensure that BAV status and configuration is confirmed. The presence of a BAV and its cusp orientation are likely to have future implications for tailoring medical and surgical treatment plans.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
OH, AB and DM contributed in data curation, project administration, methodology, conceptualisation, writing, analysis (original, review and editing). AH, BH and SO contributed in conceptualisation, supervision, project administration, writing (original, review and editing).
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Oliver James Harrison AU - Abdul Badran AU - Amer Harky AU - Daniel Muller AU - Benoy N. Shah AU - Sunil K. Ohri PY - 2020 DA - 2020/01/20 TI - Real-world Accuracy of Transthoracic Echocardiography in Diagnosing Bicuspid Aortic Valve Morphology: A Single Center UK Experience JO - Artery Research SP - 21 EP - 26 VL - 26 IS - 1 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.191219.001 DO - 10.2991/artres.k.191219.001 ID - Harrison2020 ER -
